CARUMONAM
- CAS NO.:87638-04-8
- Empirical Formula: C12H14N6O10S2
- Molecular Weight: 466.4
- MDL number: MFCD00871292
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
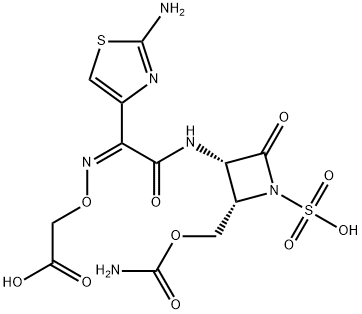
What is CARUMONAM?
Description
Canunonam is an injectable antibiotic, the second monobactam ever developed (the first being Squibb’s aztreonam). It is highly active against Gram negative bacteria, particularly pseudomom, and is reportedly effective in the treatment of pneumonia, cystitis, peritonitis and secondary infections.
Originator
Takeda (Japan)
Definition
ChEBI: An N-sulfonated monobactam antibiotic.
brand name
Amasulin
Antimicrobial activity
A synthetic monobactam with activity against common
pathogenic organisms similar to that of aztreonam. It is resistant
to hydrolysis by the common plasmid and chromosomal
β-lactamases, but it can be hydrolyzed by ESBLs.
It is administered intravenously, achieving a concentration
of c. 78 mg/L after a 20-min infusion of 1 g. The plasma halflife
is 1.7 h and the plasma protein binding 18–28%.
Carumonam is almost entirely eliminated in the glomerular
filtrate, probenecid having no effect on excretion; 96%
of labeled compound is found in the urine, with 3% in the
feces. Between 68% and 91% of the dose appears in the urine
within 24 h.
Side effects and clinical use are similar to those of
aztreonam.
Properties of CARUMONAM
| alpha | D26 -45° (c = 1 in DMSO) |
| Density | 2.13±0.1 g/cm3(Predicted) |
| pka | -0.43±0.60(Predicted) |
Safety information for CARUMONAM
Computed Descriptors for CARUMONAM
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4