Carminic Acid
Synonym(s):Carminic acid (C.I. 75470);Natural Red 4
- CAS NO.:1260-17-9
- Empirical Formula: C22H20O13
- Molecular Weight: 492.39
- MDL number: MFCD00167028
- EINECS: 215-023-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
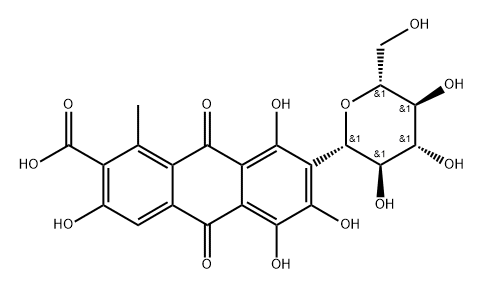
What is Carminic Acid?
Description
Carminic acid is a naturally occurring organic molecule whose structure consists of 9,10-anthraquinone-2-carboxylic acid “decorated” with a methyl group, a glucopyranose, and four hydroxyls. It is found in several species of scale insects known as cochineals, including?Dactylopius coccus?(Western Hemisphere),?Porphyrophora hamelii?(Armenia), and?Porphyrophora polonica?(north-central Europe).
In 1894, noted British dye chemist Henry Edward Schunk, working in Germany, isolated carminic acid from cochineals. German chemist Otto Dimroth reported its structure in 1920; and Indian chemists S. B. Bhatia and K. Venkataraman corrected the placement of the carboxyl group in Dimroth’s structure in 1965.
Cochineal insects use carminic acid to repel other insects. But for centuries, cochineals have been valuable to humans for providing carminic acid to use on its own or to make cochineal dyes. When carminic acid is treated with with aluminum and/or calcium salts, it forms complexes that are familiarly called carmine, carmine lake, crimson lake, or even the eponymous cochineal1. The dye is used for textiles and as a US Food and Drug Administration–approved food, cosmetic, and drug colorant, although some people exhibit allergic reactions to it.
Free carminic acid has been used as a bacteriological stain, an ingredient for artists’ paints, and a pigment for inks. In its cochineal dye form, you can even use it to?make your own colored paper?for cards and envelopes for Valentine’s Day!
1. CAS Reg. No. 1343-78-8.
Originally the Molecule of the Week for February 5, 2007
Chemical properties
red to dark red crystalline powder
Chemical properties
Cochineal extract is a concentrated solution obtained after removing alcohol from an aqueous–alcoholic extract of cochineal (Dactylopius coccus Costa, also called Coccus cati L.). This extract is used as a color additive, the primary colorant being carminic acid.
History
CI Natural Red 4(CI 75470), is a red dye occurring as a glycoside in the body of the cochineal insect Dactylopius coccus of the order Homoptera, family Coccidae. This insect is native to Central and South America. The Aztecs had extracted the dye from the insect centuries before the coming of the Spaniards. For breeding purposes, the insects were collected in the autumn and carefully protected during the winter months. Cochineal was harvested after three months, and then the bugs were killed by immersion in hot water, by placing in hot ovens, or by exposure to the hot sun. The latter method produced the highest quality dye. At present, Peru and the Canary Islands are the main source of the dye. Until the advent of synthetic dyes, the principal use for carminic acid was for dyeing tin-mordanted wool or silk. Its aluminum lake, carmine, finds use in the coloring of foods.
The Uses of Carminic Acid
Carminic acid (C.I. 75470) For staining nuclei in histological sections. Used to prepare staining solutions.
CAS 1260-17-9, pH 1.6 (10?g/l, H?O, 20?°C).
The Uses of Carminic Acid
A red glucosidal hydroxyanthrapurin, it is produced naturally within some insects as a defense mechanism.
The Uses of Carminic Acid
antineoplastic, glucosyltransferase inhibitor
What are the applications of Application
Carminic acid is an anthraquinone glucoside-containing small molecule natural product
Definition
ChEBI: A tetrahydroxyanthraquinone that is that is 1,3,4,6-tetrahydroxy-9,10-anthraquinone substituted by a methyl group at position 8, a carboxy group at position 7 and a 1,5-anhydro-D-glucitol moiety at position 2 via a C-gly osidic linkage. It is a natural dye isolated from several insects such as Dactylopius coccus.
General Description
Dark purplish-brown mass or bright red or dark red powder. Darkens at 248°F. Deep red color in water. Yellow to violet in acidic aqueous solutions.
Air & Water Reactions
Soluble in water [Hawley].
Reactivity Profile
CARMINE neutralizes bases in exothermic reactions. Incompatible with strong oxidizing agents.
Fire Hazard
Flash point data for CARMINE are not available. CARMINE is probably combustible.
Purification Methods
Carminic acid forms red prisms from EtOH. It gives a red colour in Ac2O and yellow to violet in acidic solution. UV: max (H2O) 500nm ( 6,800); (0.02N HCl) 490-500nm ( 5,800) and (0.0001N NaOH) 540nm ( 3,450). IR: max (Nujol) 1708s, 1693s, 1677m, 1648m, 1632m, 1606s, 1566s, 1509 cm-1. Periodate oxidation is complete after 4hours at 0o with the consumption of 6.2 mols. The tetra-O-methyl carminate has m 186-188o (yellow needles from *C6H6/pet ether). [IR: Ali & Haynes J Chem Soc 1033 1959, Bhatia & Venkataraman Indian J Chem 3 (2) 92 1965, Synthesis: Davis & Smith Biochemical Preparations 4 38 1955, Beilstein 18 III/1V 6697.]
Properties of Carminic Acid
| Melting point: | 136 °C |
| Boiling point: | 506.2°C (rough estimate) |
| alpha | 15654 +51.6° (water) |
| Density | 1.4504 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| Flash point: | 12℃ |
| storage temp. | room temp |
| solubility | 30g/l |
| form | Crystalline Powder |
| appearance | red crystals; bright red to dark purple powder |
| Colour Index | 75470 |
| pka | 1.62±0.20(Predicted) |
| color | Red to dark red |
| PH | 1.6 (10g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 4.8 - 6.2 |
| optical activity | [α]20/D +3.1°, c = 1 in H2O |
| Water Solubility | 1.298g/L(room temperature) |
| Merck | 14,1843 |
| BRN | 71651 |
| CAS DataBase Reference | 1260-17-9 |
| EPA Substance Registry System | C.I. Natural Red 4 (1260-17-9) |
Safety information for Carminic Acid
Computed Descriptors for Carminic Acid
Carminic Acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


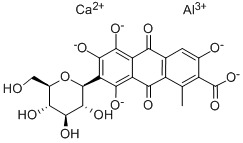


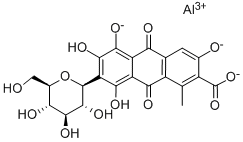
You may like
-
 1260-17-9 / 1343-78-8 Carminic acid 98%View Details
1260-17-9 / 1343-78-8 Carminic acid 98%View Details
1260-17-9 / 1343-78-8 -
 Carminic Acid (Natural dye) CAS 1260-17-9View Details
Carminic Acid (Natural dye) CAS 1260-17-9View Details
1260-17-9 -
 Carminic acid, GR CAS 1260-17-9View Details
Carminic acid, GR CAS 1260-17-9View Details
1260-17-9 -
 Carminic acid CAS 1260-17-9View Details
Carminic acid CAS 1260-17-9View Details
1260-17-9 -
 CARMINIC ACID For Microscopy CAS 1260-17-9View Details
CARMINIC ACID For Microscopy CAS 1260-17-9View Details
1260-17-9 -
 Carminic acid CAS 1260-17-9View Details
Carminic acid CAS 1260-17-9View Details
1260-17-9 -
 Carminic acid 98% CAS 1260-17-9View Details
Carminic acid 98% CAS 1260-17-9View Details
1260-17-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
