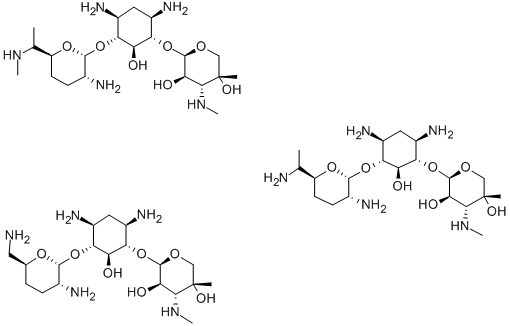
carbomycin
- CAS NO.:4564-87-8
- Empirical Formula: C42H67NO16
- Molecular Weight: 841.982
- MDL number: MFCD01716264
- SAFETY DATA SHEET (SDS)
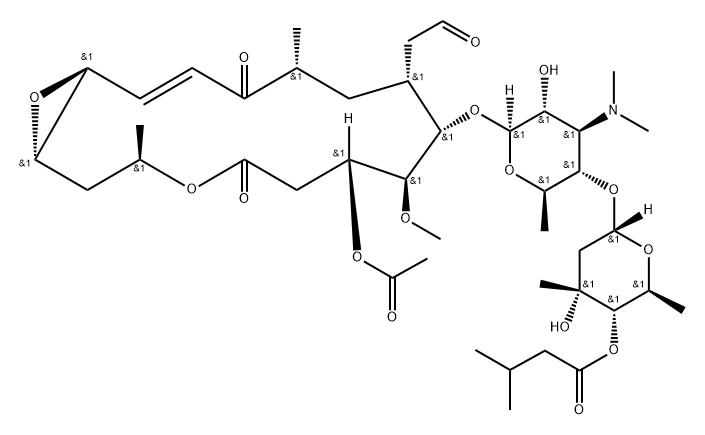
What is carbomycin?
Originator
Magnamycin,Pfizer,US,1953
The Uses of carbomycin
Sixteen-membered-ring macrolite antibiotic complex similar to Leucomycin and Erythromycin, produced by Stroptomyces halstedii.
Definition
An antibiotic isolated from products of Strepto- myces halstedii when grown in suitable media by the deep-culture method. It inhibits growth of certain gram-positive bacteria such as staphylo- cocci, pneumococci, and hemolytic streptococci.
Manufacturing Process
A selected strain of Streptomyces halstedii was cultivated in an aqueous nutrient medium under aerobic conditions and the resulting broth containing carbomycin antibiotics was filtered. The solutions was extracted twice at pH 6.5 with one-quarter volume of methyl isobutyl ketone. The combined extracts were concentrated to one-tenth volume under vacuum, and the antibiotics were extracted into water adjusted to a pH of about 2 with sulfuric acid. After adjusting the separated aqueous solution to pH 6.5, the antibiotic was extracted into benzene and the solution was concentrated to a small volume. Addition of hexane resulted in the separation of a solid product containing the benzene complexes of carbomycin A and carbomycin B, present in the fermentation broth.
brand name
Magnamycin (Pfizer).
Therapeutic Function
Antibiotic
Safety Profile
Poison by subcutaneous route. Moderately toxic by intravenous and intramuscular routes. When heated to decomposition it emits toxic fumes of NOx.
Properties of carbomycin
| Melting point: | 214° |
| Boiling point: | 769.45°C (rough estimate) |
| alpha | D25 -58.6° (chloroform) |
| Density | 1.1761 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| pka | pKb 7.2(at 25℃) |
Safety information for carbomycin
Computed Descriptors for carbomycin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1