CANNABIDIOL
Synonym(s):CBD
- CAS NO.:13956-29-1
- Empirical Formula: C21H30O2
- Molecular Weight: 314.46
- MDL number: MFCD00869597
- EINECS: 689-176-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
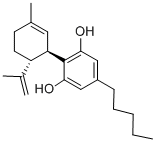
What is CANNABIDIOL?
Absorption
Following a single buccal administration, maximum plasma concentrations of both CBD and THC typically occur within two to four hours. When administered buccally,
blood levels of THC and other cannabinoids are lower compared with inhalation of smoked cannabis. The resultant concentrations in the blood are lower than those obtained by inhaling the same dose because absorption is slower, redistribution into fatty tissues is rapid and additionally some of the THC undergoes hepatic first pass metabolism to 11-OH-THC, a psycho-active metabolite.
The CBD component of sublingual Sativex was found to have a Tmax of 1.63hr and a Cmax of 2.50ng/mL, while buccal Sativex was found to have a Tmax of 2.80hr and a Cmax of 3.02ng/mL.
Description
Everyone knows (or should know) that (?)-trans-Δ9-tetrahydrocannabinol (THC) and many of its cannabinoid isomers are the chief psychoactive components of marijuana (Cannabis sativa). But some THC isomers are not psychoactive and may have some beneficial uses.
Members of the cannabidiol family fall into this category. The structure shown is the one typically used for cannabidiol; it goes by many names, including (–)-cannabidiol, Δ1(2)-trans-cannabidiol, and cannabidiol (7CI). There are at least seven known cannabidiol isomers.
In 1940, the iconic organic chemist Roger Adams and colleagues?isolated cannabidiol?as its bis(3,5-dinitrobenzoate) ester from?C. sativa, which they called “Minnesota wild hemp”. At the time, cannabidiol’s isomerism was not recognized. It was not until the 1960s that its isomers’ absolute configurations were determined and some of them were synthesized.
Israeli scientists led by Raphael Mechoulam discovered in 1970 that?cannabidiol and most other cannabinoids are not psychoactive. Since then, cannabidiol has been investigated for medical uses, including treatment for a virulent form of epilepsy. It is a component of some dietary supplements and cosmetics. Biologists are working on developing marijuana strains that suppress THC content and enhance the production of cannabidiol.
Cannabidiol’s regulatory status varies from one jurisdiction to another. The regulations are so irregular that in 2016 the European Industrial Hemp Association issued a position paper that suggested a regulatory protocol with the objective that?all European Union countries would operate under the same rules.
Description
Cannabidiol (CRM) (Item No. ISO60156) is a certified reference material categorized as a phytocannabinoid. Unlike Δ9-THC (Item Nos. ISO60157 | 12068), cannabidiol is non-psychoactive. This product is intended for research and forensic applications.
Chemical properties
Off-White Solid
The Uses of CANNABIDIOL
Major non-psychoactive constituent of Cannabis. Exhibits multiple bioactivities including anticonvulsant, anxiolytic and anti-inflammatory effects. The (+)-isomers were more active than the (-)-isomers.
Definition
ChEBI: An cannabinoid that is cyclohexene which is substituted by a methyl group at position 1, a 2,6-dihydroxy-4-pentylphenyl group at position 3, and a prop-1-en-2-yl group at position 4.
Indications
When used in combination with delta-9-tetrahydrocannabinol as the product Sativex, cannabidiol was given a standard marketing authorization (ie. a Notice of Compliance (NOC)) by Health Canada for the following indications:
1) as adjunctive treatment for symptomatic relief of spasticity in adult patients with multiple sclerosis (MS) who have not responded adequately to other therapy and who demonstrate meaningful improvement during an initial trial of therapy ;
Due to the need for confirmatory studies to verify the clinical benefit coupled with the promising nature of the clinical evidence, Sativex was also given a Notice of Compliance with Conditions (NOC/c) by Health Canada for the following indications:
1) as adjunctive treatment for the symptomatic relief of neuropathic pain in adult patients with multiple sclerosis;
2) as adjunctive analgesic treatment in adult patients with advanced cancer who experience moderate to severe pain during the highest tolerated dose of strong opioid therapy for persistent background pain .
Background
Cannabidiol, or CBD, is one of at least 85 active cannabinoids identified within the Cannabis plant. It is a major phytocannabinoid, accounting for up to 40% of the Cannabis plant's extract, that binds to a wide variety of physiological targets of the endocannabinoid system within the body. Although the exact medical implications are currently being investigated, CBD has shown promise as a therapeutic and pharmaceutical drug target. In particular, CBD has shown promise as an analgesic, anticonvulsant, muscle relaxant, anxiolytic, antipsychotic and has shown neuroprotective, anti-inflammatory, and antioxidant activity, among other currently investigated uses . CBD's exact place within medical practice is still currently hotly debated, however as the body of evidence grows and legislation changes to reflect its wide-spread use, public and medical opinion have changed significantly with regards to its usefulness in a number of medical conditions ranging from anxiety to epilepsy.
From a pharmacological perspective, Cannabis' (and CBD's) diverse receptor profile explains its potential application for such a wide variety of medical conditions. Cannabis contains more than 400 different chemical compounds, of which 61 are considered cannabinoids, a class of compounds that act upon endogenous cannabinoid receptors of the body . Cannabinoid receptors are utilized endogenously by the body through the endocannabinoid system, which includes a group of lipid proteins, enzymes, and receptors that are involved in many physiological processes. Through its modulation of neurotransmitter release, the endocannabinoid system regulates cognition, pain sensation, appetite, memory, sleep, immune function, and mood among many other bodily systems. These effects are largely mediated through two members of the G-protein coupled receptor family, cannabinoid receptors 1 and 2 (CB1 and CB2). CB1 receptors are found in both the central and peripheral nervous systems, with the majority of receptors localized to the hippocampus and amygdala of the brain. Physiological effects of using cannabis make sense in the context of its receptor activity as the hippocampus and amygdala are primarily involved with regulation of memory, fear, and emotion. In contrast, CB2 receptors are mainly found peripherally in immune cells, lymphoid tissue, and peripheral nerve terminals .
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are two types of cannabinoids found naturally in the resin of the marijuana plant, both of which interact with the cannabinoid receptors that are found throughout the body. Although THC and CBD have been the most studied cannabinoids, there are many others identified to date including cannabinol (CBN), cannabigerol (CBG), Cannabidivarin (CBDV), and Tetrahydrocannabivarin (THCV) that can be found within the medical cannabis . While both CBD and THC are used for medicinal purposes, they have different receptor activity, function, and physiological effects. If not provided in their activated form (such as through synthetic forms of THC like Dronabinol or Nabilone), THC and CBD are obtained through conversion from their precursors, tetrahydrocannabinolic acid-A (THCA-A) and cannabidiolic acid (CBDA), through decarboxylation reactions. This can be achieved through heating, smoking, vaporization, or baking of dried unfertilized female cannabis flowers.
The primary psychoactive component of Cannabis, delta 9-tetrahydrocannabinol (Δ9-THC), demonstrates its effects through weak partial agonist activity at Cannabinoid-1 (CB1R) and Cannabinoid-2 (CB2R) receptors. This activity results in the well-known effects of smoking cannabis such as increased appetite, reduced pain, and changes in emotional and cognitive processes. In contrast to THC's weak agonist activity, CBD has been shown to act as a negative allosteric modulator of the cannabinoid CB1 receptor, the most abundant G-Protein Coupled Receptor (GPCR) in the body . Allosteric regulation is achieved through the modulation of receptor activity on a functionally distinct site from the agonist or antagonist binding site which is clinically significant as direct agonists (such as THC) are limited by their psychomimetic effects such as changes to mood, memory, and anxiety.
In addition to the well-known activity on CB1 and CB2 receptors, there is further evidence that CBD also activates 5-HT1A/2A/3A serotonergic and TRPV1–2 vanilloid receptors, antagonizes alpha-1 adrenergic and μ-opioid receptors, inhibits synaptosomal uptake of noradrenaline, dopamine, serotonin and gamma-aminobutyric acid (GABA), and cellular uptake of anandamide, acts on mitochondria Ca2+ stores, blocks low-voltage-activated (T-type) Ca2+ channels, stimulates activity of the inhibitory glycine-receptor, and inhibits activity of fatty amide hydrolase (FAAH) .
CBD is currently available in Canada within a 1:1 formulation with tetrahydrocannbinol (THC) (as the formulation known as "nabiximols") as the brand name product Sativex. It is approved for use as adjunctive treatment for symptomatic relief of spasticity in adult patients with multiple sclerosis (MS). Sativex was also given a conditional Notice of Compliance (NOC/c) for use as adjunctive treatment for the symptomatic relief of neuropathic pain in adult patients with multiple sclerosis and as adjunctive analgesic treatment for moderate to severe pain in adult patients with advanced cancer .
In April 2018, a Food and Drug Administration advisory panel unanimously recommended approval of Epidiolex (cannabidiol oral solution) for the treatment of two rare forms of epilepsy - Lennox-Gastaut syndrome and Dravet syndrome, which are among the two most difficult types of epilepsy to treat . Epidiolex was granted Orphan Drug designation as well as Fast Track Approval from the FDA for further study in these hard to treat conditions. Notably, phase 3 clinical trials of Epidiolex have demonstrated clinically significant improvement in Lennox-Gastaut syndrome and Dravet syndrome . On June 25th, 2018, Epidiolex was approved by the FDA to be the first CBD-based product available on the US market.
Biological Activity
Non-psychotropic constituent of cannabis that is anticonvulsive, antihyperalgesic and neuroprotective in vivo . GPR55 and weak CB 1 antagonist (IC 50 values are 0.445 and 3.35 μ M), CB 2 receptor inverse agonist and inhibitor of anandamide uptake (IC 50 = 27.5 μ M). Also a weak agonist at VR1 vanilloid receptors (EC 50 = 3.5 μ M).
Pharmacokinetics
Although the exact mechanism and magnitude of effects of THC and CBD are not fully understood, CBD has been shown to have analgesic, anticonvulsant, muscle relaxant, anxiolytic, neuroprotective, anti-oxidant, and anti-psychotic activity. This wide variety of effects is likely due to it's complex pharmacological mechanisms. In addition to binding to CB1 and CB2 receptors of the endocannabinoid system, there is evidence that CBD activates 5-HT1A serotonergic and TRPV1–2 vanilloid receptors, antagonizes alpha-1 adrenergic and μ-opioid receptors, inhibits synaptosomal uptake of noradrenaline, dopamine, serotonin and gaminobutyric acid and cellular uptake of anandamide, acts on mitochondria Ca2 stores, blocks low-voltage-activated (T-type) Ca2 channels, stimulates activity of the inhibitory glycine-receptor, and inhibits activity of fatty amide hydrolase (FAAH) .
Pharmacology
Cannabidiol, a constituent of the cannabis plant, has been receiving considerable attention of late for its potential therapeutic utility, including potential anxiolytic, anticonvulsant,anti-inflammatory, and neuroprotective effects. Cannabidiol has a complex pharmacology. In contrast to THC, cannabidiol has minimal affinity for CB1 and CB2 receptors and produces no intoxication. The effects of cannabidiol in combination with THC have been mixed, with some data suggesting it may reduce THC’s mood-altering and cognitive effects, while others show no effect. If oral cannabidiol reduces cannabis intoxication, it could be a potential medication to treat CUD. However, Haney et al. tested a range of cannabidiol doses (200–800 mg) in combination with active and placebo cannabis and found no cannabidiol effect on the subjective, reinforcing, or cardiovascular effects of smoked cannabis, providing little support for cannabidiol’s utility as a medication to reduce cannabis’ positive reinforcing and subjective effects.
Metabolism
THC and CBD are metabolized in the liver by a number of cytochrome P450 isoenzymes, including CYP2C9, CYP2C19, CYP2D6 and CYP3A4. They may be stored for as long as four weeks in the fatty tissues from which they are slowly released at sub-therapeutic levels back into the blood stream and metabolized via the renal and biliary systems. The main primary metabolite of CBD is 7-hydroxy-cannabidiol.
Storage
-20°C
Properties of CANNABIDIOL
| Melting point: | 62-63°C |
| Boiling point: | bp2 187-190° (bath temp 220°); bp0.001 130° |
| alpha | D27 -125° (0.066 g in 5 ml 95% ethanol); D18 -129° (c = 0.45 in ethanol) |
| Density | d440 1.040 |
| refractive index | nD20 1.5404 |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Soluble to 75 mM in ethanol and to 75 mM in DMSO |
| form | powder |
| color | White |
| EPA Substance Registry System | 1,3-Benzenediol, 2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl- (13956-29-1) |
Safety information for CANNABIDIOL
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H301:Acute toxicity,oral H311:Acute toxicity,dermal H331:Acute toxicity,inhalation H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for CANNABIDIOL
| InChIKey | QHMBSVQNZZTUGM-ZWKOTPCHSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
