cangrelor tetrasodium
- CAS NO.:163706-36-3
- Empirical Formula: C17H21Cl2F3N5Na4O12P3S2
- Molecular Weight: 864.2865126
- MDL number: MFCD14635359
- EINECS: 813-784-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is cangrelor tetrasodium?
Description
Cangrelor tetrasodium is a direct purinergic platelet receptor (P2Y12) inhibitor that blocks ADP-induced platelet activation and aggregation. The drug, which was developed by The Medicine Company, binds reversibly to the P2Y12 receptor, preventing further signaling and platelet activation. Cangrelor, which was approved in June 2015 by the FDA, is indicated as an adjunct to percutaneous coronary intervention for reducing the risk of periprocedural myocardial infarction, repeat coronary revascularization, and stent thrombosis in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. The most common side effect observed with the drug was bleeding.
The Uses of cangrelor tetrasodium
Antiplatelet agent.
Definition
ChEBI: An organic sodium salt that is the tetrasodium salt of cangrelor. Used as an intravenous antiplatelet drug that prevents formation of harmful blood clots in the coronary arteries.
Synthesis
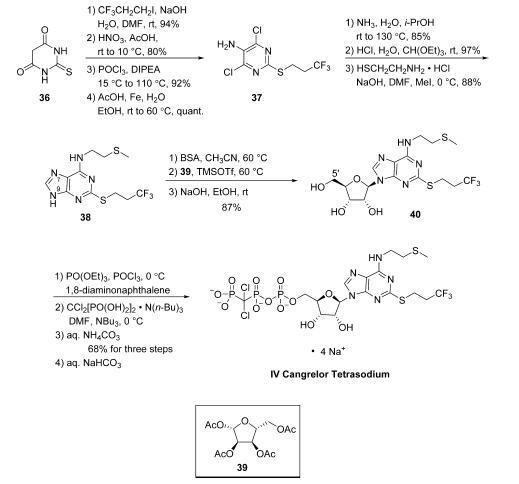
While several discovery-scale routes to cangrelor tetrasodium were previously reported,14 an improved procedure developed with the goal of providing a manufacturing scale route to cangrelor tetrasodium has recently been reported by Jinan Bestcomm Pharmaceutical R&D. Starting from commercially available 2-thiobarbituric acid (36), S-alkylation with 3,3,3-trifluoropropyl iodide proceeded in high yield (94%) under basic conditions. Nitration of this intermediate with HNO3/AcOH generated a nitro-pyrimidine diol in 80% yield. Bis-chlorination via treatment with POCl3 provided the corresponding dichloro pyrimidine (92%), and subsequent nitro reduction with AcOH/Fe under aqueous conditions yielded intermediate 37 (quantitative yield), which readily provided the bis-aniline analogue by reaction with ammonia in EtOH/H2O at 80 ??C. Condensation with triethyl orthoformate/ HCl at room temperature provided access to the desired purine in high yield (97%). A one-pot alkylation/amination strategy was then employed, first relying on S-alkylation of 2- aminoethanethiol hydrochloride with MeI/NaOH and reaction of the resulting amine with the purine chloride generated 38 in 88% across the sequence. Alkylation of 38 with commercial furanose 39 proceeded in a regioselective manner favoring N-9 functionalization, employing conditions similar to those previously described by Almond and co-workers. Toward this end, silylation of 38 with N,O-bis-(trimethylsilyl)acetamide (BSA) followed by subjection to TMSOTf and 39, resulted in the desired N-9 alkylated product, which was carried crude to global deacetylation with NaOH/EtOH at room temperature, making way for smooth conversion to alcohol 40 (87% from 38). Although phosphorylation of 40 has been performed using a variety of related methods,14 the largest scale conditions reported to date consist of initial 5?? alcohol activation with POCl3 and PO(OEt)3 in the presence of 1,8-diaminonaphthalene, furnishing the 5?? monophosphonate intermediate. This intermediate was not isolated but further treated with a solution of dichloromethylenebis(phosphonic acid) tributylammonium salt and tributylamine in DMF at 0 ??C, yielding cangrelor as a crude ammonium salt following quench with NH4HCO3.15 Purification via ion exchange chromatography provided cangrelor as its ammonium salt in 68% yield over the threestep sequence, which was subjected to aqueous NaHCO3 solution and lyophilization and provided cangrelor tetrasodium salt (IV). This synthesis was performed starting on >100 g scale of 36 and required only one chromatography step which involved ion exchange chromatography of the cangrelor ammonium salt. More recently, while beyond the scope of this article, additional reports have been disclosed describing the development of specific pharmaceutical formulations for delivery of cangrelor in high purity.
storage
Store at -20°C
Properties of cangrelor tetrasodium
| storage temp. | 4°C, away from moisture and light |
| solubility | DMSO:100.0(Max Conc. mg/mL);115.7(Max Conc. mM) |
| form | A solid |
| color | White to off-white |
| Water Solubility | Soluble to 100 mM in water |
Safety information for cangrelor tetrasodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for cangrelor tetrasodium
New Products
1-Amino-1-cyclohexanecarboxylic acid 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione S-Methylisothiosemicarbazide hydroiodide ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate Decanonitrile N,N'-diallyl-1,3-diaminopropanedihydrochloride N-(3-Nitrophenyl)cyclopropanecarboxamide (2-amino-2-phenylethyl)(methyl)amine Methyl-2-acetamidobenzoate methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 4-cyano benzaldehyde 2-hydroxy-5-bromo pyridine 5-Fluoro-2-Oxindole 3,5-Dichloro-2,6-Dimethyl-1h-Pyridin-4-One (9H-fluoren-9-yl)methyl carbonochloridate 2-methyl-5-nitroaniline (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acidRelated products of tetrahydrofuran


You may like
-
 163706-36-3 Cangrelor Tetrasodium 98%View Details
163706-36-3 Cangrelor Tetrasodium 98%View Details
163706-36-3 -
 Cangrelor tetrasodium salt CAS 163706-36-3View Details
Cangrelor tetrasodium salt CAS 163706-36-3View Details
163706-36-3 -
 4 Chloro 6,7 Dimethoxy Quinoline (Cabozantinib intermediate)View Details
4 Chloro 6,7 Dimethoxy Quinoline (Cabozantinib intermediate)View Details
35654-56-9 -
 3,4 Difluoro benzaldehydeView Details
3,4 Difluoro benzaldehydeView Details
34036-07-2 -
 10,10 Dimethyl AnthroneView Details
10,10 Dimethyl AnthroneView Details
5447-86-9 -
 Methyl 3 cyclohexene 1 carboxylateView Details
Methyl 3 cyclohexene 1 carboxylateView Details
6493-77-2 -
 Indazole 3 carboxylic acidView Details
Indazole 3 carboxylic acidView Details
4498-67-3 -
 2 Hydroxy 4 methoxy benzoic acidView Details
2 Hydroxy 4 methoxy benzoic acidView Details
2237-36-7