CALCIUM PERCHLORATE
- CAS NO.:13477-36-6
- Empirical Formula: CaCl2O8
- Molecular Weight: 238.98
- MDL number: MFCD00015971
- EINECS: 236-768-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
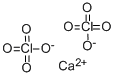
What is CALCIUM PERCHLORATE?
Description
The anhydrate is a white to yellow crystalline solid with a density of 2.651 g/cm3. Contact with this salt will irritate the eyes, skin and mucus membranes. If ingested, it is toxic in sufficient quantities. When formed from an aqueous solution, the tetrahydrate is obtained. Calcium perchlorate tetrahydrate has the formula Ca(ClO4)2·4H2O and the CAS number 15627-86-8. Its molecular weight is 311.04 g/mol and its density is 2.651 g/cm3. Its solubility in water is 188 g/ 100 ml at 20°C.
Chemical properties
white crystal(s); oxidizing agent [HAW93]
The Uses of CALCIUM PERCHLORATE
Calcium perchlorate hydrate is used as a pharmaceutical intermediate.
Preparation
Calcium perchlorate can be prepared by reacting
calcium carbonate with a solution of perchloric acid:
CaCO3+ HClO4→Ca(ClO4)2+ CO2
This salt can also be prepared by an electrochemical
method in water whereby calcium perchlorate is formed
from calcium chlorate in which a platinum anode and
a rotating stainless steel cathode is employed.
General Description
White to yellow crystalline solid. Density 2.651 g / cm3. Contact may irritate skin, eyes, and mucous membranes. May be toxic by ingestion.
Air & Water Reactions
Water soluble.
Reactivity Profile
A strong oxidizing agent. Can react with reducing agents to generate heat and products that may be gaseous (causing pressurization in closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Redox reactions can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Consequently explosive mixtures with reducing agents often persist unchanged for long periods. Such systems are typically mixtures of solids, but may involve any combination of physical states. Can react violently with active metals, cyanides, esters, and thiocyanates. May cause the acceleration of the burning of combustible materials. Prolonged exposure to heat of flames may result in an explosion.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Properties of CALCIUM PERCHLORATE
| Melting point: | decomposes at 270℃ [HAW93] |
| Density | 2.650 |
| solubility | soluble in ethanol, methanol |
| form | Crystalline |
| color | White |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| EPA Substance Registry System | Perchloric acid, calcium salt (13477-36-6) |
Safety information for CALCIUM PERCHLORATE
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P221:Take any precaution to avoid mixing with combustibles/… P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for CALCIUM PERCHLORATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
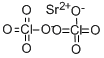
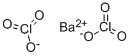
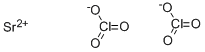
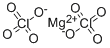
You may like
-
 Calcium perchlorate hydrate CAS 13477-36-6View Details
Calcium perchlorate hydrate CAS 13477-36-6View Details
13477-36-6 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5