Calcium chlorite
- CAS NO.:14674-72-7
- Empirical Formula: CaCl2O4
- Molecular Weight: 174.9816
- MDL number: MFCD01737662
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:54
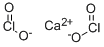
What is Calcium chlorite?
Description
Calcium chlorite is an oxysalt with a molecular formula of Ca(ClO2)2. It is a white, crystalline material that is soluble in water. It is a strong oxidizer and a fire risk in contact with organic materials. The four-digit UN identification number is 1453. Hypochlorites have two less oxygen atoms than the base-state compounds. They can cause combustion in high concentrations when in contact with organic materials. When heated or in contact with water, they can give off oxygen gas. At ordinary temperatures, they can give off chlorine and oxygen when in contact with moisture and acids. They are commonly used as bleaches and swimming pool disinfectants.
Chemical properties
White crystals. Decomposes in water.
Physical properties
Calcium chlorite, Ca(ClO2)2, has the molecular
weight of 174.98 g/mol. Its density is 2.71 g/cm3. Its
CAS number is 14674-72-7. Calcium chlorite can be
prepared by reaction of chlorous acid with calcium
carbonate:
CaCO3 (solid)+ 2HClO2 (aq)→Ca(ClO2)2·6H2O
It is a white granular solid and if formed in solution,
crystallizes as the hexahydrate. Calcium hypochlorite,
hydrated, is a white granular solid having an odor of
chlorine. It is noncombustible and accelerates the
burning of combustible materials. It is decomposed
by water with evolution of chlorine gas and heat.
Prolonged exposure to fire or heat may result in an
explosion. It is decomposed by water to form
calcium hydroxide and chlorine dioxide, an explosive
oxidizing gas:
Ca(ClO2)2+H2O→Ca(OH)2+ 2ClO2
Calcium chlorite is a strong oxidizing agent. It
ignites on contact with potassium thiocyanate and reaction
with chlorine yields explosive chlorine dioxide gas.
Reaction with ammonia produces ammonium chlorite,
which is shock sensitive and explodes. If mixed with
finely divided metallic or organic substances, the
combination is highly flammable and may be ignited
by friction.
Currently, no manufacturer is registered for the
product CALCIUM CHLORITE. There is a paucity of data concerning the physical
properties of this salt and they are not easily
accessible.
General Description
White granular solid. Prolonged exposure to fire or heat may result in an explosion and the rupturing of the containers.
Air & Water Reactions
Decomposed by water to calcium hydroxide and chlorine dioxide, an explosive oxidizing gas.
Reactivity Profile
A strong oxidizing agent. Accelerate the burning of combustible materials. Ignites on contact with potassium thiocyanate [Lewis]. Reaction with chlorine yields explosive chlorine dioxide gas. Reaction with ammonia produces ammonium chlorite, which is shock-sensitive. Finely divided mixtures with metallic or organic substances are highly flammable and may be ignited by friction (Lab. Gov. Chemist 1965).
Hazard
Strong oxidizer, fire risk in contact with organic materials.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
A strong oxidizer. Ignites on contact with potassium thiocyanate. Reaction with Cl2 yields explosive Cl0~. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORITES and CALCIUM COMPOUNDS.
Properties of Calcium chlorite
| Density | 2.710 |
| solubility | reacts with H2O |
| color | white cubic crystals, crystalline |
| Water Solubility | decomposed by H2O [HAW93] |
| CAS DataBase Reference | 14674-72-7 |
| EPA Substance Registry System | Chlorous acid, calcium salt (14674-72-7) |
Safety information for Calcium chlorite
Computed Descriptors for Calcium chlorite
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
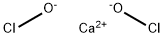
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4