BariumChlorite
- CAS NO.:14674-74-9
- Empirical Formula: BaClH3O2
- Molecular Weight: 207.8
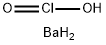
What is BariumChlorite?
Physical properties
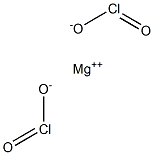
Barium chlorite, Ba(ClO2)2, is a solid with the molecular
weight of 204.779 g/mol. Its density is 3.61 g/cm3.
Its CAS number is 14674-74-9. Its melting point is
727°C and it has a boiling point of 1640°C. Barium chlorite
can be prepared by reaction of chlorous acid with
barium carbonate:
BaCO3 (s)+ 2HClO2 (aq)→Ba(ClO2)2·7H2O
It is a white granular solid and if formed in solution,
crystallizes as the heptahydrate. Like its strontium
homologue, barium chlorite is a strong
oxidizing agent. It accelerates the burning of combustible materials, ignites on contact with potassium thiocyanate
and reaction with chlorine yields explosive
chlorine dioxide gas. Reaction with ammonia
produces ammonium chlorite, which is shock sensitive.
If mixed with finely divided metallic or organic
substances, the combination is highly flammable and
may be ignited by friction.
Safety Profile
A poison. Decomposesexplosively at 190°C. Ignites on contact with dimethylsulfate. When heated to decomposition it emits toxicfumes of Cl-.
Safety information for BariumChlorite
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4