Calcium bis(cyclohexylsulphamate)
- CAS NO.:139-06-0
- Empirical Formula: C6H15CaNO3S
- Molecular Weight: 221.33
- MDL number: MFCD00064942
- EINECS: 205-349-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:14:12
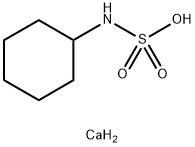
What is Calcium bis(cyclohexylsulphamate)?
Chemical properties
White crystals or powder; nearly odor- less; very sweet taste. Freely soluble in water (solu- tions are neutral to litmus); almost insoluble in alco- hol, benzene, chloroform, and ether; p H (10% solu- tion) 5.5–7.5. Sweetening power approximately 30 times that of sucrose.
Originator
Sucaryl Calcium,Abbott,US,1953
The Uses of Calcium bis(cyclohexylsulphamate)
Nonnutritive sweetener.
The Uses of Calcium bis(cyclohexylsulphamate)
Calcium bis(N-Cyclohexylsulfamate) is used in method for regulating citrus summer light by regulating cyclamate calcium nutrition.
Manufacturing Process
220 parts by weight, 2.22 mols, of cyclohexylamine and 57 parts by weight,
0,50 mol, of ammonium sulfamate were mixed at room temperature and
heated with agitation. At the end of one-half hour of heating the temperature
had reached 110°C and approximately one-half mol of ammonia had been
evolved. Heating was continued under reflux at 133°C for 22 additional hours.
A second half-mol of ammonia was liberated. The ammonia yield was 100%.
The reaction mixture was cooled to 100°C. To the mixture was added a water
slurry containing 20.3 parts by weight, 0.55 equivalent, of calcium hydroxide
and 700 parts by weight of water. Cyclohexylamine was then removed by
azeotropic distillation with water.
The amine which was recovered can be reused after drying.
The residue from the distillation was evaporated to dryness in a vacuum oven
at 50°C and the resulting product analyzed. The product weighing 105.5 parts
by weight, 0.488 equivalent, was obtained which is a 98% yield of the
technical calcium cyclohexylsulfamate dihydrate.
Therapeutic Function
Pharmaceutic aid
Hazard
Not permitted for use in foods and soft drinks, because of suspected carcinogenicity.
Safety Profile
Poison by ingestion and intravenous routes. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic and neoplastigenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SO, and NO,. See also CALCIUM COMPOUNDS.
Properties of Calcium bis(cyclohexylsulphamate)
| Merck | 13,1666 |
| EPA Substance Registry System | Calcium cyclamate(1:2) (139-06-0) |
Safety information for Calcium bis(cyclohexylsulphamate)
Computed Descriptors for Calcium bis(cyclohexylsulphamate)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4