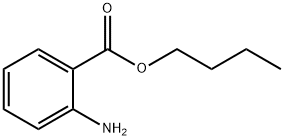
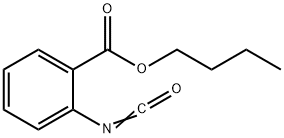
BUTYL ANTHRANILATE
- CAS NO.:7756-96-9
- Empirical Formula: C11H15NO2
- Molecular Weight: 193.24
- MDL number: MFCD00036506
- EINECS: 231-816-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-22 21:24:34
What is BUTYL ANTHRANILATE?
Chemical properties
dark brown liquid
Chemical properties
Butyl anthranilate has a sweet, faint, fruit (plum, petigrain) note.
Occurrence
Reported present in peppermint oil from Brazil, Achillea ageratum, tea and in apple aroma.
Definition
ChEBI: A benzoate ester obtained by formal condensation of the carboxy group of anthranilic acid with the hydroxy group of butanol. Found in several fruit species, it is used as a flavouring and fragrance agent and also exhibits insect repellent properties.
Preparation
Prepared by transesterification of methyl anthranilate (methyl 2-aminobenzoate) with n-butyl alcohol in the presence of HCl.
Taste threshold values
Taste characteristics at 50 ppm: floral, green, fruity, sweet citrus, waxy character.
General Description
Dark brown liquid. Insoluble in water.
Air & Water Reactions
Sensitive to air and light. Insoluble in water. BUTYL ANTHRANILATE will hydrolyze under high and low pH conditions. .
Reactivity Profile
BUTYL ANTHRANILATE is an aminophenyl ester derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Flash point data for BUTYL ANTHRANILATE are not available. BUTYL ANTHRANILATE is probably combustible.
Metabolism
Esters of benzoic acid are presumably either hydrolysed and then metabolized according to the normal pattern for the alcohol and acid produced, or possibly in some cases the ring may be hydroxylated and the product excreted as a glucuronide or sulphate ester . H-Butanol and isobutanol are rapidly oxidized in vivo, presumably to the aldehyde and acid ; only small amounts were excreted by rabbits as the glucuronic acid conjugates
Properties of BUTYL ANTHRANILATE
| Melting point: | <0 °C |
| Boiling point: | 303 °C(lit.) |
| Density | 1.06 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 2181 | BUTYL ANTHRANILATE |
| Flash point: | >230 °F |
| form | neat |
| pka | 2.17±0.10(Predicted) |
| color | A colourless or very pale straw-coloured liquid. |
| Odor | at 100.00 %. petitgrain plum sweet grape fruity wine floral berry powdery |
| JECFA Number | 1536 |
| Stability: | Air and light sensitive. Incompatible with alkalies, acids, strong oxidizing agents. |
| CAS DataBase Reference | 7756-96-9(CAS DataBase Reference) |
| EPA Substance Registry System | Butyl anthranilate (7756-96-9) |
Safety information for BUTYL ANTHRANILATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for BUTYL ANTHRANILATE
BUTYL ANTHRANILATE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 7756-96-9 n-Butyl ester 99%View Details
7756-96-9 n-Butyl ester 99%View Details
7756-96-9 -
 7756-96-9 98%View Details
7756-96-9 98%View Details
7756-96-9 -
 Butyl 2-Aminobenzoate CAS 7756-96-9View Details
Butyl 2-Aminobenzoate CAS 7756-96-9View Details
7756-96-9 -
 Butyl anthranilate CAS 7756-96-9View Details
Butyl anthranilate CAS 7756-96-9View Details
7756-96-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1