Buterizine
- CAS NO.:68741-18-4
- Empirical Formula: C31H38N4
- Molecular Weight: 466.66
- MDL number: MFCD00868271
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:41
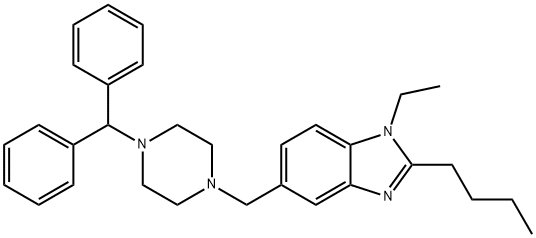
What is Buterizine?
Originator
Buterizine,Shanghai Chemfrom Chemical Co., Ltd.
The Uses of Buterizine
Vasodilator (peripheral).
Definition
ChEBI: Buterizine is a diarylmethane.
Manufacturing Process
A mixture of 10.3 parts of 1-chloro-4-(chloromethyl)-2-nitrobenzene, 25.2
parts of 1-(diphenylmethyl)piperazine and 120 parts of ethanol is stirred and
refluxed for 4 h. The reaction mixture is cooled and evaporated. The residue is
taken up in about 100 parts of water and the product is extracted with
methylbenzene. The extract is washed with water, dried, filtered and
evaporated. The residue is purified by column-chromatography over silica gel
using trichloromethane as eluent. The pure fractions are collected and the
eluent is evaporated. The residue is crystallized from a mixture of 2,2'-
oxybispropane and hexane (1:2 by volume). The product is filtered off,
washed with hexane and dried, yielding 19.6 parts of 1-(4-chloro-3-
nitrophenylmethyl)-4-(diphenylmethyl)piperazine; melting point 101.6°C.
During 20 h, gaseous ethanamine is bubbled through a stirred and hot (60-
70°C) mixture of 1-(4-chloro-3-nitrophenylmethyl)-4-
(diphenylmethyl)piperazine and dimethylsulfoxide. The reaction mixture is
cooled and poured onto ice-water. The precipitated product is filtered off,
washed with water and taken up in methylbenzene. The latter is dried, filtered
and evaporated. The residue is purified by column-chromatography over silica
gel using a mixture of trichloromethane and methanol as eluent. The pure
fractions are collected and the eluent is evaporated, yielding 4-[4-
(diphenylmethyl)-1-piperazinylmethyl]-N-ethyl-2-nitrobenzenamine; melting
point 128.2°C (crystallized from 2,2'-oxybispropane).
A solution of 4-[4-(diphenylmethyl)-1-piperazinylmethyl]-N-ethyl-2-
nitrobenzenamine in methanol is hydrogenated at normal pressure and at
room temperature with Raney-nickel catalyst. After the calculated amount of
hydrogen is taken up, the catalyst is filtered off and the filtrate is evaporated, yielding 4-[4-(diphenylmethyl)-1-piperazinylmethyl]-N1-ethyl-1,2-
benzenediamine.
A mixture of 4-[4-(diphenylmethyl)-1-piperazinylmethyl]-N1-ethyl-1,2-
benzenediamine and acetic acid is stirred at room temperature till all solid
enters solution. Then there are added pentyl ethanimidate hydrochloride and
stirring is continued first for 1 h at room temperature and further for 1 h at
reflux. The reaction mixture is evaporated and the residue is stirred in water.
The whole is alkalized with a sodium hydroxide solution and the product is
extracted with dichloromethane. The extract is dried, filtered and evaporated.
The residue is purified by column-chromatography over silica gel using a
mixture of trichloromethane and methanol as eluent. The pure fractions are
collected and the eluent is evaporated. The product is filtered off and dried,
yielding 5-[4-(diphenylmethyl)-1-piperazinylmethyl]-1-ethyl-2-butyl-1Hbenzimidazole;
melting point 124.8°C (crystallized from 4-methyl-2-
pentanone).
Therapeutic Function
Vasodilator
Safety information for Buterizine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4