bumadizone
- CAS NO.:3583-64-0
- Empirical Formula: C19H22N2O3
- Molecular Weight: 326.39
- MDL number: MFCD00864288
- EINECS: 222-710-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:15:06
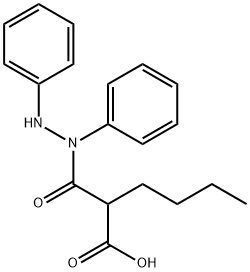
What is bumadizone ?
Originator
Eumotol,Byk Gulden,W. Germany,1972
The Uses of bumadizone
Bumadizon, is a non-steroidal anti-inflammatory (NSAID) drug.
Background
Bumadizone has been approved for use in Germany and Austria, it is a drug with anti-inflammatory, antipyretic, and analgesic properties, and was marketed for the treatment of both rheumatoid arthritis and gout . Its use is restricted to these conditions, due to risks this drug poses .
Definition
ChEBI: A carbohydrazide obtained by formal condensation of one of the carboxy groups from butylmalonic acid with the hydrazino group of 1,2-diphenylhydrazine. Used (as its calcium semihydrate) for treatment of rheumatoid arthritis.
Manufacturing Process
(a) A solution of 22.4 grams of dicyclohexylcarbodiimide in 120 ml of absolute
tetrahydrofuran is added dropwise at 5°-10°C in an atmosphere of nitrogen to
a solution of 20 grams of n-butylmalonic acid monoethyl ester and 19.6 grams
of freshly recrystallized hydrazobenzene in 320 ml of anhydrous
tetrahydrofuran. The mixture is then stirred for 15 hr at 25°C in an
atmosphere of nitrogen, then the precipitated dicyclohexyl urea is filtered off
and the filtrate, after the addition of 3 drops of glacial acetic acid, is
evaporated to dryness in vacuo. The residue is dissolved in 1 liter of ether, the
ethereal solution is extracted twice with 2 N potassium bicarbonate solution
and twice with 2 N hydrochloric acid, whereupon it is washed with water until
the washing water is neutral. The ethereal solution is dried over sodium
sulfate and concentrated in vacuo. The residue is fractionally distilled under
high vacuum whereupon the ester is obtained as a yellow oil. BP 170°C at
0.05 torr vacuum. Crystals which melt at 63°-65°C are obtained from
cyclohexane.
(b) A suspension of 7.1 grams of the ester obtained according to (a) in 40 ml
of aqueous 0.5 N sodium hydroxide solution is refluxed for 24 hours in an
atmosphere of nitrogen. The solution is filtered and traces of hydrazobenzene
are removed by extraction with ether. The aqueous solution is made acid to Congo paper at 10°C with concentrated hydrochloric acid, the oil which
separates is dissolved in 40 ml of ethyl acetate, the ethyl acetate solution is
isolated, and washed neutral with water. The solution is then extracted twice
with 36 ml of 0.5 N sodium bicarbonate solution each time.
The separate extracts are made acid to Congo paper with concentr
brand name
Bumaflex;Rheumatol.
Therapeutic Function
Analgesic, Antipyretic, Antirheumatic
World Health Organization (WHO)
Bumadizone, a pyrazolone derivative with antiinflammatory, analgesic and antipyretic activity, was introduced in 1972 for the treatment of rheumatic disorders. As it is structurally related to phenylbutazone it is subjected to rigorously restricted indications by some national regulatory authorities. See WHO comment for phenylbutazone.
Metabolism
Not Available
Properties of bumadizone
| Melting point: | 117-119℃ |
| Boiling point: | 487.4±47.0 °C(Predicted) |
| Density | 1.227±0.06 g/cm3 (20 ºC 760 Torr) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 3.69±0.21(Predicted) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for bumadizone
Computed Descriptors for bumadizone
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![calcium bis[2-butyl-3-(1,2-diphenylhydrazino)-3-oxopropionate]](https://img.chemicalbook.in/CAS/GIF/34461-73-9.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4