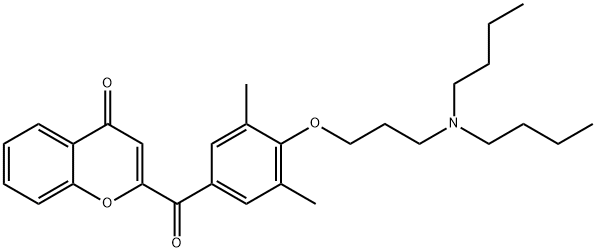
Bucromarone
- CAS NO.:78371-66-1
- Empirical Formula: C29H37NO4
- Molecular Weight: 463.61
- MDL number: MFCD00864709
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:42
What is Bucromarone?
Originator
Bucromarone,Transphyto
The Uses of Bucromarone
Bucromarone is an antiarrhythmic drug.
The Uses of Bucromarone
Cardiac depressant (anti-arrhythmic).
Definition
ChEBI: Bucromarone is an aromatic ketone.
Manufacturing Process
1.2 mol of an electrophilic catalyst, advantageously aluminum chloride, was
slowly added to a solution of 48.8 g (0.4 mol) of 2,6-dimethyl phenol in 400
ml of dichloroethane and agitated at room temperature for an hour. The
mixture was cooled to 0°C and a solution of 84.0 g (0.4 mol) of 2-carboxylic
chromone acid chloride in 400 ml of a dichloroethane, was slowly added.
Agitation was continued at 0°C for 5 h and then at room temperature for 4
days. The reaction mixture was poured into 1.6 L of iced 50% hydrochloric
acid. The resulting precipitate was filtered, washed with water, dried and
106.0 g (72% yield) of 2-(3,5-dimethyl-4-hydroxybenzoyl)chromone, melting
point 216°C (recrystallised from dioxane) were obtained.
6.9 g (0.05 mol) of potassium carbonate was added to a solution of 29.4 g
(0.1 mol) of 2-(3,5-dimethyl-4-hydroxybenzoyl)chromone in 250 ml of
dimethyl formamide and kept at 100°C for an hour. A solution of 20.5 g (0.1
mol) of 3-N,N-dibutylamino-1-chloropropane in 100 ml of diethyl formamide
was then added and the resulting solution heated to 130°C for 3 h. The
solution was filtered, the dimethyl formamide was concentrated, dissolved in
300 ml water and extracted twice with 200 ml of benzene. The organic phase
was dried on magnesium sulfate and the hydrochloride was precipitated by
adding hydrochloric ether. 40.0 g (yield 80%) of 2-[4-(3-N,N-dibutylaminopropoxy)-
3,5-dimethylbenzoyl]chromone were obtained (recrystallised from an
acetone/ether mixture).
Therapeutic Function
Antiarrhythmic, Coronary vasodilator
Safety information for Bucromarone
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![4-[3-(Dimethylamino)propoxy]benzaldehyde](https://img.chemicalbook.in/CAS/GIF/26934-35-0.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1