BUCINDOLOL
- CAS NO.:70369-47-0
- Empirical Formula: C22H25N3O2
- Molecular Weight: 0
- MDL number: MFCD00865898
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
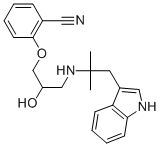
What is BUCINDOLOL?
Originator
Bucindolol hydrochloride,Bristol Myers (BMS)
The Uses of BUCINDOLOL
Antihypertensive.
Manufacturing Process
A solution of 2-cyanophenol (25.0 g, 0.21 mole), epichlorohydrin (117.0 g,
1.26 mole), and piperidine (10 drops) was stirred and heated at 115-120°C in
an oil bath for 2 h. The reaction mixture was then concentrated (90/30 mm)
to remove unreacted epichlorohydrin. The residue was diluted with toluene
and taken to dryness twice to help remove the last traces of volatile material.
The residual oil was dissolved in 263 ml of THF, and the solution stirred at 40-
50°C for 1 h with 263 ml of 1 N NaOH. The organic layer was separated and
concentrated to give an oil which was combined with the aqueous phase. The
mixture was extracted with CH2Cl2 and the extract dried (MgSO4) and
concentrated to give 36.6 g (100%) of 2-[(2,3-epoxy)propoxy]benzonitrile as
oil, which slowly crystallized to a waxy solid.
A mixture of gramine (120.0 g, 0.69 mole), 2-nitropropane (443 ml), and
NaOH (28.8 g, 0.72 mole) was stirred and gradually heated to reflux under
N2. After a 6.5 h reflux period, the reaction mixture was allowed to stand at
room temperature overnight, and then diluted with 600 ml of 10% aqueous
AcOH. The mixture was extracted with 1.5 l of Et2O and the organic layer
washed with H2O (4x 500 ml). Concentration of the Et2O solution in vacuum
gave an oil which was dissolved in 500 ml of 95% EtOH. This solution was
diluted with 300 ml of H2O. After cooling, the yellow solid was collected on a
filter to give 105.0 g (70%) of nitro intermediate, melting point 72-74°C. The
nitro compound was dissolved in 1.3 L of 95% EtOH, and Raney nickel
(70.0 g, EtOH-washed) and added. The mixture was heated to reflux, and a
solution of 85% hydrazine hydrate (116.0 g, 2.3 mole) in 95% EtOH (110 ml)
was added dropwise at a rate to maintain gentle reflux. The mixture was then
heated at reflux for an additional 1.5 h, cooled, and filtered. Concentration of
the filtrate gave crude product as a solid. A solution of the solid in 400 ml of
EtOAc was diluted with 500 ml of (i-Pr)2O and cooled. The white, cottony solid
which separated was collected on a filter to give 91.0 g (100%) of 2-(3-
indolyl)-1,1-dimethylethylamine, melting point 122-126°C.
A solution of 2-[(2,3-epoxy)propoxy]benzonitrile (18.3 g, 0.10 mole) and 2-
(3-indolyl)-1,1-dimethylethylamine (15.2 g, 0.08 mole), in 500 ml of abs.
EtOH was stirred at reflux overnight. After concentration of the reaction
mixture to approximately 200 ml and seeding, crude product began to
precipitate. The mixture was then cooled and the precipitate separated by
filtration to give 24.8 g of the free base form of the product, white solid,
melting point 120-123°C. The crude solid was dissolved in 400 ml of boiling
MeOH, and the solution was cooled with stirring, as a by-product 1,1’-[[1,1-
dimethyl-2-(1H-indol-3-yl]ethyl]imino]bis-[3-(2-cyanophenoxy)-2-propanol]
precipitated. The by-product was collected on a filter and air dried to give 2.2
g, of 2-[2-hydroxy-3-[[2-(3-indolyl)-1,1-dimethylethyl]amino]propoxy]
benzonitrile, melting point 180-187°C.
In practice it is usually used as hydrochloride.
Therapeutic Function
Antihypertensive
Properties of BUCINDOLOL
| Melting point: | 185-187° |
| pka | 8.86(at 25℃) |
Safety information for BUCINDOLOL
Computed Descriptors for BUCINDOLOL
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8