BRYOSTATIN 1
Synonym(s):Bryo 1, PKC Activator VII, PKC Activator VI;Bryostatin 1 - CAS 83314-01-6 - Calbiochem;NSC 339555
- CAS NO.:83314-01-6
- Empirical Formula: C47H68O17
- Molecular Weight: 905.03
- MDL number: MFCD00893832
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
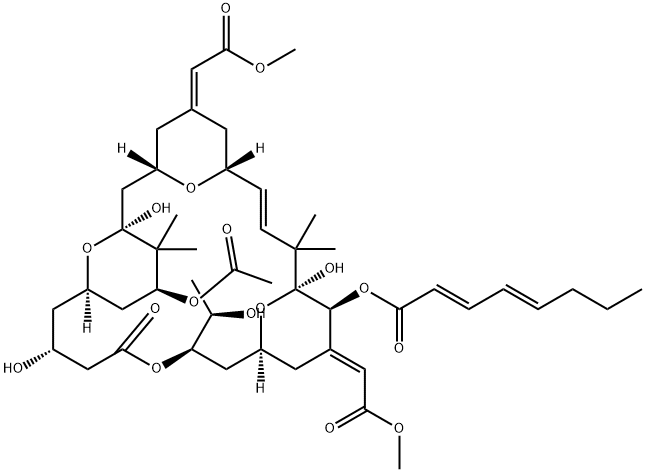
What is BRYOSTATIN 1?
Description
Macrolide lactones are natural products that have large, usually 14- to 16-membered, rings as their basic structures. They often have saccharide substituents, as in the antibiotics erythromycin and azithromycin.
The bryostatins are exceptions. They have 20-membered rings; and none of their substituents are saccharides. They occur in?Bugula neritina,?a species of tiny sea animals in the phylum Bryozoa. More than 20 bryostatins have been identified.
In 1960, chemist George Pettit, then at the University of Maine (Orono) isolated bryostatin 1 from?B. neritina. Its structure was established in 1982; and subsequent research showed that it is a potent protein kinase C modulator. In clinical trials, it has shown activity against conditions such as cancer, HIV/AIDS, and Alzheimer’s disease.
It takes ≈1 t of bryozoans to isolate 1 g of bryostatin 1, so clearly an efficient lab synthesis is needed to pursue additional research. This year, Paul A. Wender and colleagues at Stanford University reported the shortest synthesis yet (29 steps!) of bryostatin 1. The previous record was almost twice that number. The compound is approved for use in human trials, but Wender believes that recently synthesized analogues may have greater medical value.
The Uses of BRYOSTATIN 1
Bryostatin 1 has been used to study its effects on spontaneous Crohn′s disease (CD) like colitis in?mice. It has also been used to study its effects as an anthelmintic drug on adult Syphacia muris infection in rats.
What are the applications of Application
Bryostatin 1 is a macrolactone marine natural product that binds to and modulates PKC
Definition
ChEBI: A member of the class of bryostatins that is (17E)-2-oxooxacyclohexacos-17-ene which is substituted by hydroxy groups at positions 4, 10, and 20; an acetoxy group at position 8; methyl groups at positions 9, 9, 18, and 19; 2-methoxy-2-oxoe hylidene groups at positions 14 and 24; an (E,E)-octa-2,4-dienoyloxy group at position 21; and with oxygen bridges linking positions 6 to 10, 12 to 16, and 20 to 24. It is one of the most abundant member of the class of br ostatins.
Biological Activity
Protein kinase C (PKC) activator that binds with high affinity (K i = 1.35 nM). Initially activates and subsequently induces downregulation of PKC isozymes. Sensitizes tumor cells to cytotoxic effects of anticancer agents.
Biochem/physiol Actions
Bryostatin 1 (Bry1) is a macrocyclic lactone. This synaptogenic compound is obtained from the marine bryozoan?Bugula neritina. Bry1 is capable of reversing synaptic loss. It can enable synaptic maturation in animal models with several neurological disorders. It possesses antidepressant activity, when administered intracebroventricularly. Bry1 is known to participate in protecting cell tight junctions (TJs), anti-inflammatory functions and immune regulation.
storage
Store at -20°C
References
[1]. gschwendt m, fürstenberger g, rose-john s, et al. bryostatin 1, an activator of protein kinase c, mimics as well as inhibits biological effects of the phorbol ester tpa in vivo and in vitro. carcinogenesis, 1988, 9(4): 555-562.
[2]. stone rm, sariban e, pettit gr, et al. bryostatin 1 activates protein kinase c and induces monocytic differentiation of hl-60 cells. blood, 1988, 72(1): 208-213.
[3]. schuchter lm, esa ah, may s, et al. successful treatment of murine melanoma with bryostatin 1. cancer res, 1991, 51(2): 682-687.
Properties of BRYOSTATIN 1
| Melting point: | 230-235° |
| Boiling point: | 716.44°C (rough estimate) |
| alpha | D25 +34.1° (c = 0.044 in methanol) |
| Density | 1.1245 (rough estimate) |
| refractive index | 1.5940 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| form | solid |
| pka | 11.10±0.70(Predicted) |
| appearance | white solid |
| color | white |
Safety information for BRYOSTATIN 1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. |
Computed Descriptors for BRYOSTATIN 1
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Bryostatin 1 CAS 83314-01-6View Details
Bryostatin 1 CAS 83314-01-6View Details
83314-01-6 -
 Bryostatin 1 CAS 83314-01-6View Details
Bryostatin 1 CAS 83314-01-6View Details
83314-01-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1