Brompheniramine
- CAS NO.:86-22-6
- Empirical Formula: C16H19BrN2
- Molecular Weight: 319.24
- MDL number: MFCD00865691
- EINECS: 201-657-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
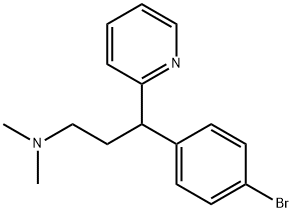
What is Brompheniramine?
Absorption
Antihistamines are well absorbed from the gastrointestinal tract after oral administration.
Toxicity
Oral, rat: LD50 = 318 mg/kg. Signs of overdose include fast or irregular heartbeat, mental or mood changes, tightness in the chest, and unusual tiredness or weakness.
The Uses of Brompheniramine
Brompheniramine is also used for allergy symptoms, rhinitis, and dermatitis. Its activity is approximately the same as that of chlorpheniramine. Synonyms of this drug are dimetane, brombey, spentan, veltane, and others.
The Uses of Brompheniramine
antidepressant
Indications
For the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing.
What are the applications of Application
Brompheniramine is a reuptake inhibitor of seratonin
Background
Histamine H1 antagonist used in treatment of allergies, rhinitis, and urticaria.
Definition
ChEBI: Pheniramine in which the hydrogen at position 4 of the phenyl substituent is substituted by bromine. A histamine H1 receptor antagonist, brompheniramine is used (commonly as its maleate salt) for the symptomatic relief of allergic cond tions, including rhinitis and conjunctivitis.
brand name
Dimetane (Wyeth).
Pharmacokinetics
Brompheniramine is an antihistaminergic medication of the propylamine class. It is a first-generation antihistamine. In allergic reactions an allergen interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Brompheniramine is a histamine H1 antagonist (or more correctly, an inverse histamine agonist) of the alkylamine class. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies.
Metabolism
Hepatic (cytochrome P-450 system), some renal.
Properties of Brompheniramine
| Melting point: | 25°C |
| Boiling point: | bp0.5 147-152° |
| Density | 1.3740 (rough estimate) |
| refractive index | 1.5770 (estimate) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| pka | pKa 3.59 (Uncertain) |
| color | Colourless |
| CAS DataBase Reference | 86-22-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Brompheniramine(86-22-6) |
| EPA Substance Registry System | 2-Pyridinepropanamine, .gamma.-(4-bromophenyl)-N,N-dimethyl- (86-22-6) |
Safety information for Brompheniramine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Brompheniramine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 86-22-6 -3-(4-bromophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine 99%View Details
86-22-6 -3-(4-bromophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine 99%View Details
86-22-6 -
 86-22-6 98%View Details
86-22-6 98%View Details
86-22-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
