BROMOCHLORODIFLUOROMETHANE
- CAS NO.:353-59-3
- Empirical Formula: CBrClF2
- Molecular Weight: 165.36
- MDL number: MFCD00042122
- EINECS: 206-537-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
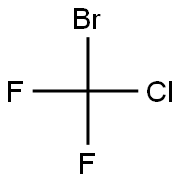
What is BROMOCHLORODIFLUOROMETHANE?
Description
Chlorodifluorobromomethane is a colorlessgas or liquid under pressure. Molecular weight=165.4;Boiling point=2 4℃; Freezing/Meltingpoint=2160.5℃. Hazard Identification (based on NFPA704 M Rating System): Health 2, Flammability 0,Reactivity 0. Insoluble in water
Chemical properties
Chlorodifluorobromomethane is a colorless gas or liquid under pressure.
Definition
ChEBI: A one-carbon compound that is methane in which the hydrogens have been replaced by two fluorines, a bromine, and a chlorine. Widely used in 'vapourising liquid'-type fire extinguishers, its use is now generally banned under the Montreal Protocol (ozone-dep eting substances), although it is still used in certain applications (e.g. aviation).
General Description
BROMOCHLORODIFLUOROMETHANE is a colorless, nonflammable gas. BROMOCHLORODIFLUOROMETHANE is mildly toxic by inhalation. BROMOCHLORODIFLUOROMETHANE can asphyxiate by the displacement of air. Exposure of the container to prolonged heat or fire may cause BROMOCHLORODIFLUOROMETHANE to rupture violently and rocket. BROMOCHLORODIFLUOROMETHANE is used as a refrigerant gas.
Reactivity Profile
BROMOCHLORODIFLUOROMETHANE is chemically inert in many situations, but can react violently with strong reducing agents such as the very active metals and the active metals. Can react with strong oxidizing agents or weaker oxidizing agents under extremes of temperature.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Mutation data reported. An asphyxiant. See also ARGON for description of inert gas asphyxiants. When heated to decomposition it emits very toxic fumes of Br-, Cl-, and F-
Potential Exposure
Used as a refrigerant and fire extinguishing agent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Iffrostbite has occurred, seek medical attention immediately;do NOT rub the affected areas or flush them with water. Inorder to prevent further tissue damage, do NOT attempt toremove frozen clothing from frostbitten areas. If frostbitehas NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
Storage
Nonflammable gas. Color Code—Green: Generalstorage may be used. Prior to working with this chemicalyou should be trained on its proper handling and storage.Before entering confined space where this chemical maybe present, check to make sure sufficient oxygen (19%)exists. Store in tightly closed containers in a cool, wellventilated area away from heat and sparks. Procedures forthe handling, use, and storage of cylinders should be incompliance with OSHA 1910.101 and 1910.169, as withthe recommendations of the Compressed Gas Association.
Shipping
UN1974 Chlorodifluorobromomethane or Refrigerant gas R-12B1, Hazard Class: 2.2; Labels: 2.2- Nonflammable compressed gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
The liquefied gas poured into water may be violently explosive. This is due to the phase transition from superheated liquid to vapor. Chlorodifluorobromomethane is generally chemically inert; however, it can react violently with strong reducing agents such as hydrides and highly active metals. It will react with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides strong oxidizing agents. It can also react with weak oxidizers under extreme temperatures.
Waste Disposal
Return refillable compressed gas cylinders to supplier.
Properties of BROMOCHLORODIFLUOROMETHANE
| Melting point: | -160,5°C |
| Boiling point: | -3,7°C |
| Density | 1,85 g/cm3 |
| refractive index | 1.3371 (estimate) |
| Water Solubility | 15.8mg/L(0 ºC) |
| EPA Substance Registry System | Halon 1211 (353-59-3) |
Safety information for BROMOCHLORODIFLUOROMETHANE
Computed Descriptors for BROMOCHLORODIFLUOROMETHANE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
