Bromobenzene
Synonym(s):Bromobenzene;Phenyl bromide
- CAS NO.:108-86-1
- Empirical Formula: C6H5Br
- Molecular Weight: 157.01
- MDL number: MFCD00000055
- EINECS: 203-623-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
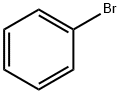
What is Bromobenzene?
Description
Bromobenzene is a flammable, clear, colorlessmobile liquid with a pleasant odor. Molecularweight=157.02. Specific gravity (H2O:1)=1.48; Boilingpoint=156℃; Freezing/Melting point=2 31℃; Flashpoint=51℃; Autoignition temperature=565℃. Explosivelimits in air: LEL=6%; UEL=36.5%[icsc]. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 2, Reactivity 0. Very slightly soluble in water; solubility=0.04% at 25℃.
Chemical properties
Bromobenzene, also known as phenyl bromide and monobromobenzene, is a benzene halogen derivative generated by benzene being substituted by bromine in the presence of iron powder. The chemical reaction principle is the same as that of chlorination of benzene to produce chlorobenzene. The reaction between bromobenzene and metal magnesium can generate phenylmagnesium bromide, which is a Grignard reagent with important uses in organic synthesis.
Physical properties
Mobile, clear, colorless to pale yellow liquid with an aromatic odor. The reported odor threshold is 4.6 ppm (Mateson, 1955). Insoluble in water, soluble in benzene, alcohol, ether, chlorobenzene and other organic solvents. It is irritating to the skin and anesthetic to the nerves. Its toxicity is stronger than chlorobenzene. Inhalation of its vapors can cause anemia and damage the liver.
The Uses of Bromobenzene
Bromobenzene is a colorless, flammable liquid with a density greater than water and with an aromatic odor. It is synthesized by the reaction of bromide with benzene in the presence of iron powder. It is used for organic synthesis, particularly in the production of the intermediate phenylmagnesium bromide. Bromobenzene is an additive to motor oils and used as a crystallizing solvent. It is used as an ingredient in the manufacture of phencyclidine, a recreational drug.
What are the applications of Application
Bromobenzene is an aryl halide used to introduce a phenyl group
Definition
ChEBI: Bromobenzene is the simplest member of the class of bromobenzenes, that is benzene in which a single hydrogen has been substituted by a bromine. A liquid at room temperature (m.p. -30℃; b.p.760 156℃), it is used as a solvent, particularly for large-scale crystallisations, and for the introduction of phenyl groups in organic synthesis. It has a role as a non-polar solvent, a hepatotoxic agent and a mouse metabolite. It is a member of bromobenzenes, a bromoarene and a volatile organic compound.
What are the applications of Application
The compound is employed as a starting material in organic syntheses in which a Grignard intermediate (phenyl magnesium bromide) is used. The material is a chemical precursor for certain agricultural products and has been used as an additive to motor oils. Bromobenzene has also been used as a high-density solvent for chemical recrystallization processes. especially for crystallizations on a large scale and where a heavy liquid is desirable.
Preparation
Bromobenzene is obtained by reacting benzene with bromine. First add iron powder and benzene into the reactor, slowly add bromine under stirring, keep the reaction at 70-80°C for 1 h after adding, the obtained crude product is washed with water and 5% sodium hydroxide solution, left to stand for stratification, distillation, Drying, filtering, and finally fractional distillation under constant pressure, taking the fraction at 155-157°C to obtain the finished product.
Synthesis Reference(s)
The Journal of Organic Chemistry, 30, p. 304, 1965 DOI: 10.1021/jo01012a512
Tetrahedron Letters, 26, p. 1935, 1985 DOI: 10.1016/S0040-4039(00)98345-X
General Description
Mobile clear colorless liquid with a pungent odor. Flash point 124°F. Denser than water and insoluble in water. Hence sinks in water. Vapors are heavier than air. A skin irritant.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Bromobenzene may be sensitive to light. May react with oxidizing agents .
Health Hazard
Contact with liquid causes irritation of eyes and mild irritation of skin. Ingestion causes mild irritation of mouth and stomach.
Fire Hazard
Moderate fire risk. Special Hazards of Combustion Products: Irritating hydrogen bromide and other gases may be produced in fire.
Biochem/physiol Actions
Bromobenzene induces hepatic necrosis via the formation of a reactive metabolite that arylates vital cellular macromolecules.
Safety Profile
Moderately toxic by ingestion, subcutaneous, and intraperitoneal routes. LWdly toxic by inhalation. An eye and mucous membrane irritant. Mutation data reported. Flammable liquid when exposed to heat, sparks, or flame. Can react with oxidtzing materials. To fight fire, use water to blanket fire, foam, CO2, water spray or mist, dry chemical. Violent reaction with bromobutane + sodium when heated above 30℃. When heated to decomposition it emits toxic fumes of Br-. See also BROMIDES.
Potential Exposure
Mutagen.Bromobenzene is used as an intermediate in organic synthesis,and as an additive in motor oil and fuels. During chlorination water treatment, bromobenzene can be formed insmall quantities.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Source
No MCLs, MCLGs, or DWELs have been proposed (U.S. EPA,
2000).
Storm water runoff, waste motor oils, improper disposal of laboratory solvent containing
bromobenzene (quoted, Verschueren, 1983)
Environmental Fate
Bromobenzene will volatilize from dry surfaces, due to its vapor pressure of 4.18mmHg at 25°C, and therefore will exist as a vapor in the environment. Bromobenzene will undergo little hydrolysis in water and little biodegradation by aquatic microorganisms. Bromobenzene is not expected to adsorb to sediment from water due to its soil sorption constant (Koc) of 150 and water solubility of 446 mg l-1. It is also expected to have a high mobility in soil and volatilize easily from moist surfaces due to its Henry’s law constant of 2.47×10-3 atmm3 mol-1 at 25°C. Bioconcentration factors range from low values of 8.8 in carp to moderately high values of 190 in algae.
Metabolic pathway
Bromobenzene and chlorobenzene are metabolized by human and mouse hepatic microsomes to two different epoxide intermediates, which rearrange to form either o- or p-bromo- and o- or p-chlorophenols, respectively. Humans preferentially metabolize halobenzenes through the hepatotoxic 3,4-epoxide pathway, suggesting that humans may be more susceptible than mice to halobenzene-induced hepatotoxicity.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with bromobenzene you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a refrigerated area away from incompatiblematerials listed above. Protect from light. Metal containers involving the transfer of this chemical should begrounded and bonded. Drums must be equipped with selfclosing valves, pressure vacuum bungs, and flame arresters.Use only nonsparking tools and equipment, especiallywhen opening and closing containers of this chemical.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or storedin a manner that could create a potential fire or explosionhazard.
Shipping
Bromobenzene requires a “FLAMMABLELIQUID” label. It falls in Hazard Class 3 and PackingGroup II.
Purification Methods
Wash bromobenzene vigorously with conc H2SO4, then 10% NaOH or NaHCO3 solutions, and H2O. Dry it with CaCl2 or Na2SO4, or pass it through activated alumina, before refluxing with, and distilling from, CaH2, using a glass helix-packed column. [Beilstein 5 IV 670.]
Toxicology
The acute toxicity of bromobenzene is lowin test animals. The toxic symptoms includesomnolence, respiratory stimulation, and muscle contraction. The oral LD50 value in rats is2700 mg/kg.
Incompatibilities
Forms explosive mixture with air.Incompatible with strong oxidizers, alkaline earth metals(barium, calcium, magnesium, strontium, etc.), metallicsalts; with risk of violent reactions. May accumulate staticelectrical charges; may cause ignition of its vapors.
Properties of Bromobenzene
| Melting point: | -31 °C |
| Boiling point: | 156 °C(lit.) |
| Density | 1.491 g/mL at 25 °C(lit.) |
| vapor density | 5.41 (vs air) |
| vapor pressure | 10 mm Hg ( 40 °C) |
| refractive index | n |
| Flash point: | 124 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with diethyl ether, alcohol, carbon tetrachloride, chloroform and benzene. |
| form | Liquid |
| color | Clear colorless to faintly yellow |
| Odor | Pleasant. |
| explosive limit | 0.5-2.5%(V) |
| Water Solubility | insoluble. <0.1 g/100 mL at 20.5 ºC |
| Merck | 14,1406 |
| BRN | 1236661 |
| Henry's Law Constant | 2.47 at 25 °C (gas stripping-GC, Shiu and Mackay, 1997) |
| Dielectric constant | 5.4(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 108-86-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, bromo-(108-86-1) |
| EPA Substance Registry System | Bromobenzene (108-86-1) |
Safety information for Bromobenzene
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Bromobenzene
| InChIKey | QARVLSVVCXYDNA-UHFFFAOYSA-N |
Bromobenzene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Bromobenzene 99%View Details
Bromobenzene 99%View Details -
 108-86-1 Bromobenzene 98%View Details
108-86-1 Bromobenzene 98%View Details
108-86-1 -
 Bromobenzene extrapure AR CAS 108-86-1View Details
Bromobenzene extrapure AR CAS 108-86-1View Details
108-86-1 -
 Bromobenzene 99% CAS 108-86-1View Details
Bromobenzene 99% CAS 108-86-1View Details
108-86-1 -
 Industrial Grade Bromobenzene Cas 108 86 1, LiquidView Details
Industrial Grade Bromobenzene Cas 108 86 1, LiquidView Details
108-86-1 -
 BromobenzeneView Details
BromobenzeneView Details
108-86-1 -
 Industrial Grade BromobenzeneView Details
Industrial Grade BromobenzeneView Details
108-86-1 -
 Liquid Bromobenzene (108-86-1), Packaging Type: Drum, Packaging Size: 25 LitreView Details
Liquid Bromobenzene (108-86-1), Packaging Type: Drum, Packaging Size: 25 LitreView Details
108-86-1
