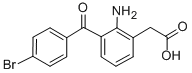
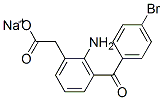
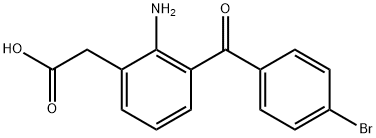
Bromfenac sodium
- CAS NO.:91714-94-2
- Empirical Formula: C15H12BrNO3
- Molecular Weight: 334.16
- MDL number: MFCD00864341
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
What is Bromfenac sodium?
Absorption
The plasma concentration of bromfenac following ocular administration in humans is unknown.
Originator
Duract,Wyeth-Ayerst
The Uses of Bromfenac sodium
Analgesic.
The Uses of Bromfenac sodium
Bromfenac (Xibrom, ISTA Pharmaceuticals, Irvine, USA; Bronuck, Senju Pharmaceutical, Osaka, Japan) is indicated for the treatment of postoperative inflammation and the reduction of ocular pain in patients after undergoing cataract extraction. For this task, one drop of Xibrom may be applied to the affected eye twice daily beginning 24 hours after cataract surgery and continuing for the first 2 weeks of the postoperative period. The clinical safety and efficacy of bromfenac have been extensively studied in diverse comparative investigations, including the treatment of external or anterior ocular inflammatory diseases, allergic conjunctivitis, scleritis, and postoperative inflammation.The results of two phase III multicenter, randomized double-masked placebo-controlled clinical trials showed that bromfenac ophthalmic solution 0.09% was effective in the rapid resolution of ocular pain after cataract surgery, and there was a statistically significant difference between the bromfenac and placebo groups demonstrated in these phase III clinical trials.
Background
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Ophthalmic NSAIDs are becoming a cornerstone for the management of ocular pain and inflammation. Their well-characterized anti-inflammatory activity, analgesic property, and established safety record have also made NSAIDs an important tool for optimizing surgical outcomes. Non-ophthalmic formulations of bromfenac were withdrawn in the US in 1998 due to cases of severe liver toxicity.
Indications
For the treatment of postoperative inflammation in patients who have undergone cataract extraction.
Definition
ChEBI: Amfenac in which the the hydrogen at the 4 position of the benzoyl group is substituted by bromine. It is used for the management of ocular pain and treatment of postoperative inflammation in patients who have undergone cataract extraction. It was withdraw from the US market in 1998, following concerns over off-label abuse and hepatic failure.
Manufacturing Process
Reaction of (2-aminophenyl)-(4-bromophenyl)-methanone with
methylsulfanylacetic acid ethyl ester and tert-butyl hypochlorite gives a
corresponding sulfonium salt. This salt was transformed to initially to the
betaine. Electrocyclic rearrangement of that transient intermediate leads, after
rearomatization, to the homoanthranilic acid. Internal ester-amine interchange
leads then to 4-bromophenyl-(3-(methylthio)indolin-7-yl)methanone. The
thiomethyl group is then removed with Raney nickel to give 4-bromophenyl-
(indolin-7-yl)methanone. Saponification of this intermediate affords the (2-
amino-3-(4-bromobenzoyl)-phenyl)-acetic acid (Bromfenac).
In practice it is usually used as sodium salt.
brand name
Xibrom (Ista).
Therapeutic Function
Analgesicá Antiinflammatory
Pharmacokinetics
Bromfenac ophthalmic solution is a sterile, topical, nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use.
Pharmacology
The drug is rapidly absorbed and excreted. The drug’s long duration of anti-inflammatory action despite its short half-life deserves further investigation.
Clinical Use
Bromfenac, used as the sodium salt, is a non-steroidal anti-inflammatory drug acting via inhibition of COX-1 and COX-2. It was launched in Japan in 2000 by Senju for topical treatment of ocular inflammation and has been approved in the United States in 2006 for the treatment of pain following cataract surgery. It was used for the short-term treatment of acute pain, but it was withdrawn for this indication in June 1998 because of several postmarketing reports of severe hepatic failure
Synthesis
The cyclization of 2-amino-4’-
bromobenzophenone with ethyl 2-(methylthio)
acetate with tert-butyl hypochlorite as
catalyst in dichloromethane at 70 ?C gives 7-
(4-bromobenzoyl)-3-(methylthio)-2,3-dihydro-
1H-indol-2-one, which is desulfurized by treatment
with Raney nickel in THF yielding 7-(4-
bromobenzoyl)-2,3-dihydro-1H-indol-2-one.
Finally, this compound is hydrolyzed with refluxing
3 M aqueous NaOH and acidified with
concentrated HCl .
Veterinary Drugs and Treatments
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) by virtue of its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Bromfenac is indicated for treatment of postoperative inflammation in patients who have undergone cataract extraction.
Metabolism
Not Available
Properties of Bromfenac sodium
| Boiling point: | 562.2±50.0 °C(Predicted) |
| Density | 1.565±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 4.07±0.10(Predicted) |
Safety information for Bromfenac sodium
Computed Descriptors for Bromfenac sodium
Bromfenac sodium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Bromfenac 98%View Details
Bromfenac 98%View Details -
 BROMFENAC 99%View Details
BROMFENAC 99%View Details -
 Bromfenac 99%View Details
Bromfenac 99%View Details
91714-94-2 -
 Bromfenac 91714-94-2 98%View Details
Bromfenac 91714-94-2 98%View Details
91714-94-2 -
 Bromfenac 98% CAS 91714-94-2View Details
Bromfenac 98% CAS 91714-94-2View Details
91714-94-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6