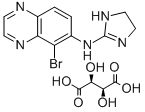
Brimonidine
Synonym(s):5-Bromo-N-(2-imidazolin-2-yl)-6-quinoxalinamine;5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine;Brimonidine
- CAS NO.:59803-98-4
- Empirical Formula: C11H10BrN5
- Molecular Weight: 292.13
- MDL number: MFCD00153878
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34

What is Brimonidine?
Absorption
Brimonidine readily penetrates the cornea following ocular administration to reach pharmacologically active concentrations in the aqueous humor and ciliary body, the putative sites of its IOP-lowering activity. Following ocular administration of 0.2% brimonidine solution, the peak plasma concentrations were achieved within 1 to 4 hours.
In a clinical study of adult subjects with facial erythema of rosacea, brimonidine was cutaneously applied on facial skin in a repeated manner. While there was no drug accumulation in plasma, the highest peak plasma concentrations (Cmax) and AUC were 46 ± 62 pg/mL and 417 ± 264 pgxhr/mL, respectively.
Toxicity
LD50 and Overdose
Oral LD50 is 50 mg/kg in mice and 100 mg/kg in rats. While there is limited clinial data on brimonidine overdose in adults, some common symptoms from oral overdoses of alpha-2 adrenergic agonists include hypotension, asthenia, vomiting, lethargy, sedation, bradycardia, arrhythmias, miosis, apnoea, hypotonia, hypothermia, respiratory depression and seizure. Treatment of an oral overdose includes supportive and symptomatic therapy. Cases of brimonidine overdose have been reported in neonates, infants, and pediatric patients receiving brimonidine tartrate as part of medical treatment of congenital glaucoma or by accidental oral ingestion. In these cases, children experienced symptoms consistent with previously reported oral overdoses of alpha-2 adrenergic agonists in young children.
Nonclinical Toxicology
At oral doses of up to 2.5 and 5 mg/kg/day in pregnant rats and rabbits, brimonidine was not shown to be teratogenic during gestation days 6 through 18. Findings from various in vitro and in vivo studies, including the Ames bacterial assay, CHO cell chromosomal aberration assay, and CD-1 mice studies, did not demonstrate any mutagenic or clastogenic potential of brimonidine. There were no observable adverse effects on male or female fertility when tested at oral doses of up to 1 mg/kg, which is approximately 200 times the systemic exposure following the maximum recommended ophthalmic dose of 0.5% brimonidine.
Use in special populations
Due to limited clinical data on the use of brimonidine pregnant or breastfeeding female patients, the use of brimonidine in these patients is generally not recommended and the use should be only considered after taking into account the benefit-to-risk ratio of continuing the drug therapy in these patients. In nursing mothers, the decision should be made whether to discontinue the drug or discontinue breastfeeding. As the systemic absorption and elimination of brimonidine are not significantly affected by age, the use of brimonidine is considered safe in geriatric patients. In contrast, the use of brimonidine in infants under the age of 2 and pediatric patients under the age of 18 is strongly not recommended due to the reports of serious adverse events following ophthalmic administration of brimonidine in infants between the age of 28 days and 3 months.
Description
Launched in the US for open-angle glaucoma and ocular hypertension, brimonidine is a relatively selective and potent α2a,-adrenergic agonist with low affinity for the imidazoline l1 receptor. Topical application reduces intraocular pressure. This bypasses any central hypotensive effects at the l1 receptor (which can also give rise to a decrease in blood pressure and heart rate) if given systemically, since topical application results in low plasma levels concomitant with rapid systemic elimination. Brimonidine can be prepared in a four-step sequence from 4-nitrophenylenediamine.
Chemical properties
Yellow Solid
Originator
Allergan (USA)
The Uses of Brimonidine
a2-Adrenoceptor agonist. Antiglaucoma
The Uses of Brimonidine
α2-Adrenoceptor agonist. Antiglaucoma.
The Uses of Brimonidine
coronary vasodilator calcium ion influx inhibitor
Background
Brimonidine is an alpha-adrenergic agonist and 2-imidazoline derivative that was first introduced in 1996. It is considered to be a third generation alpha-2 aadrenergic receptor agonist, since it displays preferential binding at alpha-2 adrenoceptors over alpha-1 receptors. Brimonidine displays a higher selectivity toward the alpha-2 adrenergic receptors than clonidine or apraclonidine, which are also alpha-2 adrenergic agonists. Alpha-2 adrenergic agonists are members of the ocular hypotensive agent drug class that are used in the chronic treatment of glaucoma. Early treatment and management of glaucoma, which predominantly involves the lowering of intraocular pressure, is critical since glaucoma is considered to be a common cause of blindness worldwide.
Ophthalmically, brimonidine is used to lower intraocular pressure by reducing aqueous humor production and increasing uveoscleral outflow. Because it is oxidately stable, brimonidine is associated with fewer reports of ocular allergic reactions compared to other alpha-2 adrenergic agonists. The ophthalmic solution of brimonidine was first approved by the FDA in 1996 as Alphagan and brimonidine is the only selective alpha-adrenergic receptor agonist approved for chronic treatment in glaucoma. Brimonidine is also found in ophthalmic solutions in combination with brinzolamide under the market name Simbrinza for the reduction in intraocular pressure. Unlike nonselective beta-blockers used in ocular hypertension, brimonidine is not associated with significantly adverse cardiopulmonary side effects. Thus brimonidine is an effective and safe alternative to beta-blockers, in patients with, or at high risk for, cardiopulmonary disease. The topical form of brimonidine was approved by the FDA in August 2013 for the symptomatic treatment of persistent facial erythema of rosacea in adults. It is marketed under the brand name Mirvaso. Brimonidine is the first topical treatment approved for facial erythema of rosacea.
What are the applications of Application
UK 14,304 is an α2-adrenergic receptor agonist
Indications
Opthalmic
Indicated for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension as monotherapy or combination product with brinzolamide.
Topical
Indicated for the treatment of persistent (non-transient) facial erythema of rosacea in adults 18 years of age or older.
Definition
ChEBI: Brimonidine is a quinoxaline derivative, a secondary amine and a member of imidazoles. It has a role as an adrenergic agonist, an antihypertensive agent and an alpha-adrenergic agonist.
Manufacturing Process
6-Aminoquinoxaline (2.08 g, 14.4 mmol) was dissolved in 11.5 ml glacial
acetic acid. The solution was cooled in water while a solution of bromine (0.74
ml, 2.3 g, 14.4 mmol) in 1.5 ml glacial acetic acid was added slowly over 15
min. After stirring for an additional 30 min. the orange red solid formed was
filtered off and washed thoroughly with dry ether. The solid was dried in vacuo
overnight to yield 4.44 g crude product (a yield of 100%). The compound, 6-
amino-5-bromoquinoxaline hydrobromide, had no definite melting point. A phase change (from fine powder to red crystals) was noticed at about 220°C.
Decomposition was observed at about 245°C. It was used directly for the next
step.
The crude 6-amino-5-bromoquinoxaline from above was dissolved in water
and saturated sodium bisulfite solution was added until the resulting solution
tested negative with starch-iodide paper. The solution was then basified with 2
N sodium hydroxide and extracted thoroughly with ethyl acetate. The organic
extract was dried over magnesium sulfate and concentrated under reduced
pressure to give the free base. The crude product was recrystallized from
boiling benzene to give yellow crystals, m.p. 155°-156°C. Using various
analytical procedures, the yellow crystals were determined to be 6-amino-5-
bromoquinoxaline. The yield was 82%.
The crude hydrobromide product previously noted (4.27 g, 14.0 mmol) was
dissolved in 60 ml of water and thiophosgene (1.28 ml, 16.8 mmol) was
added in small portions with vigorous stirring. After 2 hours, the red color of
the solution was discharged. The solid formed was filtered off and washed
thoroughly with water. After drying in vacuo at 25°C 3.38 g (a yield of 90%)
of brick red crystals was obtained, m.p. 157°-158°C. A portion of this material
was further purified by column chromatography to give white crystals, m.p.
157°-158°C. Using various analytical procedures, these crystals were
determined to be 5-bromo-6-isothiocyanatoquinoxaline.
A solution of the isothiocyanate (3.25 g, 12.2 mmol) in 145 ml benzene was
added to a solution of ethylenediamine (5.43 g, 90.0 mmol) in 18 ml benzene
at 25°C over 2 hours. After stirring for a further 30 min., the supernatant was
poured off. The oil which remained was washed by swirling with dry ether
three times and used directly for the next step. A portion of this product was
further purified by column chromatography (SiO2, CHCl3) for characterization.
A white solid was decomposed at 175°C. This white solid was determined to
be 5-bromo-6-(N-2-(aminoethyl)thioureido)quinoxaline.
The crude product from above was dissolved in 100 ml dry methanol and the
brown solution was refluxed for 19 hours until hydrogen sulfide gas was no
longer evolved. The mixture was cooled to room temperature and
concentrated to about 50 ml. The yellow solid was filtered off and dried in
vacuo; weight 2.52 g (a yield of 70%), m.p. 242°-244°C. As the crude
product was insoluble in most common organic solvents, initial purification
was achieved by an acid-base extraction procedure. 23 g of the crude product
was dissolved in 100 ml 0.5 N hydrochloric acid. The turbid yellow solution
was filtered to give a clear orange yellow solution which was extracted twice
with ethyl acetate (2x10 ml). The aqueous phase was cooled to 0°C and
basified with 6 N sodium hydroxide, keeping the temperature of the solution
below 15°C at all times. The yellow solid which precipitated was filtered off
and washed thoroughly with water until the washings were neutral to pH
paper. The solid was dried overnight in vacuo to give 1.97 g yellow solid, m.p.
249°-250°C. The recovery was about 88%.
Further purification was achieved by recrystallization as described below. The
partially purified product from above was dissolved in N,N-dimethylformamide
(about 17 ml/g) at 100°C with vigorous stirring. The solution was filtered hot
and set aside to cool overnight. The bright yellow crystals were collected by
filtration, m.p. 252°-253°C. Recovery was from 65-77%. Using various analytical procedures the bright yellow solid was determined to be 5-bromo-6-
(2-imidazolin-2-ylamino)quinoxaline.
brand name
Alphagan (Allergan).
Therapeutic Function
Antiglaucoma
Hazard
A poison by ingestion.
Biological Activity
Full α 2 adrenergic agonist. Centrally active following systemic administration in vivo . Also available as part of the α 2 -Adrenoceptor Tocriset™ and Mixed Adrenergic Tocriset™ .
Biochem/physiol Actions
UK 14,304 is an α2-adrenoceptor agonist. UK 14,304 inhibits hormone-sensitive lipase (HSL) activity and suppresses lipogenesis in adipose tissue.
Pharmacokinetics
Brimonidine is a highly selective alpha-2 adrenergic receptor agonist that is 1000-fold more selective for the alpha2-adrenergic receptor than the alpha1-adrenergic receptor. This characteristic gives the drug some therapeutic advantages, since it reduces the risk of systemic side effects, such as systemic hypotension, bradycardia, and sedation. In addition, there is a reduction in the risk for developing alpha-1 mediated ocular unwanted effects, such as conjunctival blanching, mydriasis, and eyelid retraction. However, despite high alpha-2 receptor specificity, brimonidine may still produce alpha-1 adrenoceptor-mediated ocular effects, such as conjunctival vasoconstriction. Brimonidine has a peak ocular hypotensive effect occurring at two hours post-dosing. In a randomized, double-blind clinical study, ocular administration of 0.2% brimonidine in healthy volunteers resulted in a 23% reduction of mean intraocular pressure from baseline at 3 hours following administration. In comparative studies consisting of patients with open-angle glaucoma or ocular hypertension, the ocular hypotensive effect of brimonidine was maintained during treatment periods of up to 1 year.
Brimonidine mediates vasoconstrictive effects and it was shown to exhibit anti-inflammatory properties in ex vivo human skin model and in vivo inflammation models. In a clinial trials consisting of adults with moderate to severe facial erythema of rosacea, brimonidine was shown to improve the extent of redness at 3 hours after application, compared to placebo. It was shown to be a potent vasoconstrictor of human subcutaneous vessels with a diameter of less than 200 μm. In in vivo mouse inflammation models, brimonidine displayed anti-inflammatory properties by inhibiting edema. In a randomized, double-blind study, brimonidine reduced erythema for the 12 hours of the study in a dose-dependent manner.
When adminsitered systemically, brimonidine was shown to cause cardiovascular effects by decreasing blood pressure, decreasing heart and respiratory rate, and prolonging the PR interval in the electrocardiogram. This is due to the targeting of adrenoceptors by the drug. Although the clinical significance has not been established, there is evidence that brimonidine exhibits neuroprotective activity in experimental models of cerebral ischemia and optic nerve injury. In vitro studies show that brimonidine mediated protective effects on neuronal cells from kainate acid insult and on cultured retinal ganglion cells from glutamate-induced cytotoxicity, which is a possible mediator of secondary neuronal degeneration in human glaucoma. Neuroprotective actions of brimonidine were also demonstrated in rat models of acute retinal ischemia and chronic IOP elevation. It has been proposed that brimonidine may exert neuroprotective effects on the retina and optic nerve by enhancing intrinsic retinal ganglion cell survival mechanisms and/or induction of neuronal survival factors, such as bFGF. However, further investigations are needed to conclude on these possible therapeutic benefits of the drug.
Veterinary Drugs and Treatments
Brimonidine is an alpha-adrenergic receptor agonist. It has a peak ocular hypotensive effect occurring at two hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and by and increasing uveoscleral outflow. After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours. In humans, systemic metabolism of brimonidine is extensive. It is metabolized primarily by the liver. Urinary excretion is the major route of elimination of the drug and its metabolites. Approximately 87% of an orally-administered radioactive dose was eliminated within 120 hours, with 74% found in the urine.
Metabolism
Brimonidine is reported to be metabolized in the cornea. Brominidine that reaches the systemic circulation upon topical administration undergoes extensive hepatic metabolism mediated by hepatic aldehyde oxidases.
Storage
Store at RT
Properties of Brimonidine
| Melting point: | 207.5 °C |
| Boiling point: | 432.6±55.0 °C(Predicted) |
| Density | 1.82±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: <0.8 mg/mL |
| form | powder to crystal |
| pka | 7.69±0.10(Predicted) |
| color | Light yellow to Amber to Dark green |
| Merck | 14,1375 |
| CAS DataBase Reference | 59803-98-4(CAS DataBase Reference) |
Safety information for Brimonidine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Brimonidine
Brimonidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Brimonidine 98%View Details
Brimonidine 98%View Details -
 59803-98-4 98%View Details
59803-98-4 98%View Details
59803-98-4 -
 Brimonidine CAS 59803-98-4View Details
Brimonidine CAS 59803-98-4View Details
59803-98-4 -
 Brimonidine 98% CAS 59803-98-4View Details
Brimonidine 98% CAS 59803-98-4View Details
59803-98-4 -
 UK 14,304 CAS 59803-98-4View Details
UK 14,304 CAS 59803-98-4View Details
59803-98-4 -
 Monoclonal Anti-UBB antibody produced in mouse CASView Details
Monoclonal Anti-UBB antibody produced in mouse CASView Details -
 Brimodin Eye Drop (Brimonidine)View Details
Brimodin Eye Drop (Brimonidine)View Details
59803-98-4 -
 Brimonidine, Packaging Size: 25 Kg, Packaging Type: HDPE BagView Details
Brimonidine, Packaging Size: 25 Kg, Packaging Type: HDPE BagView Details
70359-46-5
