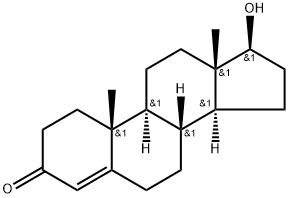
Bolasterone
- CAS NO.:1605-89-6
- Empirical Formula: C21H32O2
- Molecular Weight: 316.48
- EINECS: 216-519-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:59
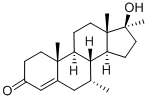
What is Bolasterone?
Originator
Myagen,Upjohn
The Uses of Bolasterone
Synthetic anabolic; epimeric with Calusterone (C148900). Controlled substance (anabolic steroid).
Definition
ChEBI: Bolasterone is a 3-hydroxy steroid. It has a role as an androgen.
Manufacturing Process
A mixture of 0.4 g of cuprous chloride, 20 ml of 4 M methylmagnesium
bromide in ether and 60 ml of redistilled tetrahydrofuran was stirred and
cooled in an ice bath during the addition of a mixture of 2.0 g of 6-dehydro-
17-methyltestosterone, 60 ml of redistilled tetrahydrofuran and 0.2 g of
cuprous chloride. The ice bath was removed and stirring was continued for 4
h. Ice and water were than carefully added, the solution acidified with 3 N
hydrochloric acid and extracted several times with ether. The combined ether
extracts were washed with a brine-sodium carbonate solution, brine and then g column of magnesium silicate (Florisil) packed wet with hexanes
(Skellysolve B). The column was eluted with 250 ml of hexanes, 0.5 liter of
2% acetone, two liters of 4% acetone and 3.5 L of 6% acetone in hexanes.
The residues from fractions 8 to 16 were combined and rechromatographed
over a 125.0 g column of magnesium silicate. The column was eluted with 6%
acetone in hexanes. Fractions 18 to 29 were combined and dissolved in
acetone, decolorized with charcoal, and recrystallized from acetone. 1.0 g of a
crystalline mixture of the 7-epimers of 7,17-dimethyltestosterone was
obtained melting at 120° to 140°C.
The 7α-isomer are separated according to following procedure:
To obtain the 7(α)-isomer of 7,17-dimethyltestosterone the crystalline mixture
of the 7 stereoisomers of 7,17-dimethyltestosterone was refluxed in tertiary
butyl alcohol with recrystallized chloranil under nitrogen. The reaction mixture
was concentrated under a fast stream of nitrogen, diluted with methylene
chloride and the solution washed with dilute sodium hydroxide, water and
then dried, filtered and the solvent removed. The residue, was combined with
the product from an identical run and chromatographed through a magnesium
silicate column developed with solvent of the following composition and order:
two each of hexane hydrocarbons (Skellysolve B), hexanes plus 4% acetone,
hexanes plus 8% acetone, hexanes plus 12% acetone, hexanes plus 14%
acetone, hexanes plus 16% acetone, hexanes plus 18% acetone, hexanes
plus 20% acetone, hexanes plus 24% acetone, hexanes plus 28% acetone,
and two of acetone.
The residues, eluted with mixture: water-acetone, were combined and
chromatographed through a 50 g 1:1 charcoal (Darco)-diatomaceous earth
(Celite) column. The column was developed with solvent of the following
composition and order: methanol, a 1:1 mixture of methanol and acetone, a
1:2 mixture of methanol and acetone, acetone and a 1:4 mixture of acetone
and methylene chloride. Fractions, containing 7(α)-epimer were combined, the
solvent evaporated and the residue crystallized from acetone to give the
7α,17-dimethyltestosterone, melting point at 163° to 165°C.
Therapeutic Function
Anabolic
Properties of Bolasterone
| Melting point: | 163-165° |
| Boiling point: | 441.0±45.0 °C(Predicted) |
| Density | 1.09±0.1 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), Dioxane (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 15.13±0.70(Predicted) |
| color | Off-White to Pale Yellow |
Safety information for Bolasterone
Computed Descriptors for Bolasterone
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8