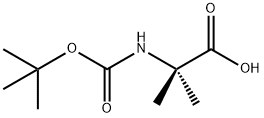
Boc-N-methyl-L-leucine
Synonym(s):Boc-N-methyl-L -leucine;Boc-N-Me-Leu-OH;N-α-t.-Boc-N-α-methyl-L-leucine
- CAS NO.:53363-89-6
- Empirical Formula: C12H23NO4
- Molecular Weight: 245.32
- MDL number: MFCD00038522
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:52
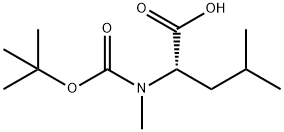
What is Boc-N-methyl-L-leucine?
Description
Boc-N-methyl-L-leucine is the BOC (tert-butyloxycarbonyl) protected N-methylated leucine. The N-methylated amino acid is quite useful in the peptide synthesis, giving a lot of improved properties to peptide. Studies have shown that peptide containing N-methylated amino acids obtain increased proteolytic stability, increased membrane permeability, and altered conformation. The effects of N-methylated amino acids are quite important in the development of therapeutic peptide compound as well as in the assay of biological activity of some modified peptides. BOC group can be easily removed by treatment with strong acids such as trifluoroacetic acid.
The Uses of Boc-N-methyl-L-leucine
Boc-N-methyl-L-leucine, is an amino acid building block used in peptide synthesis. With a growing peptide drug market the fast, reliable synthesis of peptides is of great importance.
Biological Activity
Boc-N-methyl-L-leucine is a peptidyl nucleobase that has been shown to inhibit tumor cell growth and induce tumor regression in animal models. It is an amide of leucine and the antibiotic aminouracil, which is a guanine analogue. Boc-N-methyl-L-leucine has also shown to inhibit tumor growth by inhibiting mitochondrial membrane potential, which may lead to cell death in cancer cells. This compound has been shown to be effective against both solid tumors and leukemia cell lines. Boc-N-methyl-L-leucine also inhibits the proliferation of human prostate cancer cells by blocking the synthesis of nucleic acids and proteins.
References
https://en.wikipedia.org/wiki/Tert-Butyloxycarbonyl_protecting_group
http://pubs.acs.org/doi/abs/10.1021/cr030024z?src=recsys&journalCode=chreay
Properties of Boc-N-methyl-L-leucine
| Melting point: | 55-60 °C |
| Boiling point: | 338.2±21.0 °C(Predicted) |
| Density | 1.053±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| pka | 4.04±0.21(Predicted) |
| form | Solid |
| color | White to off-white |
| optical activity | [α]20/D 36±2°, c = 1% in methylene chloride |
| BRN | 2373250 |
| InChI | InChI=1S/C12H23NO4/c1-8(2)7-9(10(14)15)13(6)11(16)17-12(3,4)5/h8-9H,7H2,1-6H3,(H,14,15)/t9-/m0/s1 |
| CAS DataBase Reference | 53363-89-6(CAS DataBase Reference) |
Safety information for Boc-N-methyl-L-leucine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Boc-N-methyl-L-leucine
| InChIKey | YXJFAOXATCRIKU-VIFPVBQESA-N |
| SMILES | C(O)(=O)[C@H](CC(C)C)N(C(OC(C)(C)C)=O)C |
Boc-N-methyl-L-leucine manufacturer
Nandana Bioscience (OPC) Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Boc-N-methyl-L-leucine CAS 53363-89-6View Details
Boc-N-methyl-L-leucine CAS 53363-89-6View Details
53363-89-6 -
 53363-89-6 Boc-N-Me-Leu-OH 98%View Details
53363-89-6 Boc-N-Me-Leu-OH 98%View Details
53363-89-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
