BLEOMYCIN A2
- CAS NO.:11116-31-7
- Empirical Formula: C55H84N17O21S3+
- Molecular Weight: 1415.55
- MDL number: MFCD00866496
- EINECS: 2343565
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:31
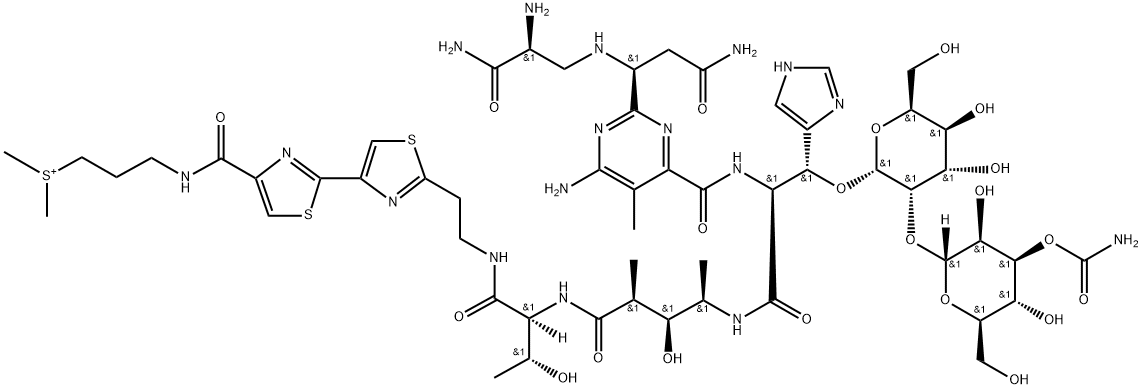
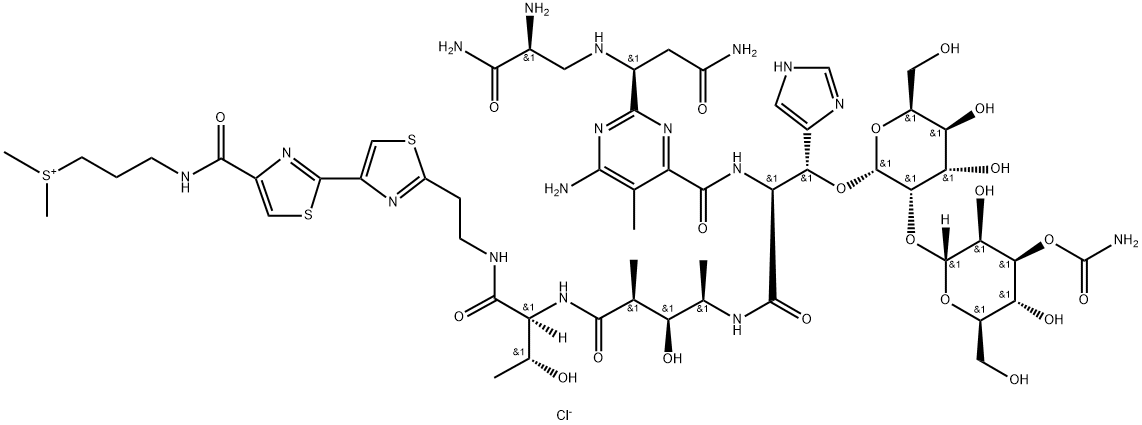
What is BLEOMYCIN A2?
Description
The term “bleomycin” represents a group of glycopeptides used as cancer drugs and antibiotics. It was discovered in 1962 by microbiologist Hamao Umezawa at the Institute of Microbial Chemistry (Tokyo), who observed that culture filtrates of the bacterium Streptomyces verticillus had anticancer activity.
All bleomycins have the same general structure; they differ only by the functional group attached to the terminal amine. The most prominent member, bleomycin A2 (shown), contains a (dimethylsulfonio)propyl cation on the amine. It is marketed in the form of a sulfate salt that contains other bleomycins. The total synthesis of bleomycin A2 was reported in 1981 by Umezawa and colleagues at several Japanese research institutions. The commercial product, however, is obtained from S. verticillus.
Bleomycin sulfate is used primarily to treat Hodgkin and non-Hodgkin lymphoma; testicular and cervical cancers; squamous cell carcinomas; and cancer-related pleural effusion. It is administered via intravenous and intramuscular injection.
Biochemist JoAnne Stubbe at MIT (Cambridge, MA) was awarded the 2020 Priestley Medal for her work on enzyme mechanisms and for uncovering the details of the mechanism of action of bleomycin, which cleaves double-stranded DNA. Stephen J. Lippard, also at MIT, calls Stubbe “the top mechanistic biochemist of her generation”. The Priestley Medal for lifetime achievement in chemistry is the highest award given by ACS.
Definition
ChEBI: Bleomycin A2 is a bleomycin. It has a role as an antineoplastic agent and a metabolite.
Properties of BLEOMYCIN A2
| Melting point: | 200–204 oc (dec.)a |
| solubility | 20 g/la |
| appearance | white to yellowish powdera |
Safety information for BLEOMYCIN A2
Computed Descriptors for BLEOMYCIN A2
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![2-[2-(3-aminopropoxy)ethoxy]ethanol](https://img.chemicalbook.in/CAS/GIF/112-33-4.gif)

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
