Bis(pinacolato)diboron
Synonym(s):4,4,4′,4′,5,5,5′,5′-Octamethyl-2,2′-bi-1,3,2-dioxaborolane
- CAS NO.:73183-34-3
- Empirical Formula: C12H24B2O4
- Molecular Weight: 253.94
- MDL number: MFCD00799570
- EINECS: 615-925-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-26 21:25:56
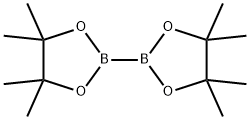
What is Bis(pinacolato)diboron?
Description
Bis(pinacolato)diboron is typically used for making pinacol boronate esters. These boronates are generally used subsequently in palladium catalyzed Suzuki couplings. They are also sometimes used in other reactions such as oxidations to form phenols. Pinacol boronate esters are generally easier to handle than boronic acids.
Chemical properties
Bis(pinacolato)diboron is a white crystal powder, It is commonly used coupling reagent.
The Uses of Bis(pinacolato)diboron
Bis(pinacolato)diboron is used as a condensation agent in the preparation poly(arylene)s. Bis(pinacolato)diboron is an organoboranate with potential use as matrix metallo-proteinase (MMP-2) inhibitor.
The Uses of Bis(pinacolato)diboron

The Iodo compound (308 mg, 0.513 mmol), the boronic ester (194 mg, 0.641 mmol), K3PO4 (327 mg, 1.539 mmol), XPhos (14.67 mg, 0.031 mmol), and Pd2(dba)3 (14.09 mg, 0.015 mmol) were combined in a microwave vial. The vial was purged with argon, after which time was added dioxane (3 mL) and H2O (0.5 mL) The reaction was irradiated in a microwave reactor at 120 C for 10 min. Additional boronic ester (50 mg) was added and the mixture was irradiated at 120 C for another 10 min. The mixture was then treated with aq 1N NaOH and extracted with EtOAc (50 mL). The org layer was dried (MgSO4), concentrated, and purified by flash chromatography (20% MeOH/DCM) to provide the product as a dark yellow solid. [177 mg, 53%]
The Uses of Bis(pinacolato)diboron
suzuki reaction
The Uses of Bis(pinacolato)diboron
As a boron source in Pt-mediated diborations, coupling reactions; in Rh-or Ir-mediated borylations of alkanes and arenes, and in carbenoid chemistry.
What are the applications of Application
Bis(pinacolato)diboron is a reagent used for the cis-vicinal diborylation of acetylenes and olefins with Pt catalysis; borylation of aromatics by Pd catalysis
Definition
ChEBI: Bis(pinacolato)diboron is a 1,3,2-dioxaborolane that is 4,4,5,5-tetramethyl-1,3,2-dioxaborolane in which the boron hydrogen at position 2 is replaced by a 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl group. It is a reagent used in organic synthesis to synthesise pinacol boronic esters. It has a role as an EC 3.4.24.24 (gelatinase A) inhibitor.
General Description
Bis(pinacolato)diboron or (B2pin2) is the most commonly used diborane reagent in organic synthesis due to its high stability in air and moisture. It can be synthesized by treating tetrakis(dimethylamino)diboron with pinacol in acidic conditions.
Properties of Bis(pinacolato)diboron
| Melting point: | 137-140 °C(lit.) |
| Boiling point: | 222.6±7.0 °C(Predicted) |
| Density | 0.98 |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| appearance | Colorless solid |
| form | Powder or Platelets |
| color | White |
| Water Solubility | Soluble in tetrahydrofuran, dichloromethane, toluene, hexane and heptane. Insoluble in water. |
| Sensitive | moisture sensitive |
| Merck | 14,1300 |
| BRN | 7703552 |
| CAS DataBase Reference | 73183-34-3(CAS DataBase Reference) |
Safety information for Bis(pinacolato)diboron
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Bis(pinacolato)diboron
| InChIKey | IPWKHHSGDUIRAH-UHFFFAOYSA-N |
Bis(pinacolato)diboron manufacturer
HYCHEM LABORATORIES
ASM Organics
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran





You may like
-
 BIS(PINACOLOTO) DIBORANE 73183-34-3 98%View Details
BIS(PINACOLOTO) DIBORANE 73183-34-3 98%View Details
73183-34-3 -
 73183-34-3 99%View Details
73183-34-3 99%View Details
73183-34-3 -
 Bis(pinacolato)diboron CAS 73183-34-3View Details
Bis(pinacolato)diboron CAS 73183-34-3View Details
73183-34-3 -
 Bis(pinacolato)diboron CAS 73183-34-3View Details
Bis(pinacolato)diboron CAS 73183-34-3View Details
73183-34-3 -
 Bis(pinacolato)diboron, 99% CAS 73183-34-3View Details
Bis(pinacolato)diboron, 99% CAS 73183-34-3View Details
73183-34-3 -
 Bis(pinacolato)diboron CAS 73183-34-3View Details
Bis(pinacolato)diboron CAS 73183-34-3View Details
73183-34-3 -
 Bis(pinacolato)diboron 98% CAS 73183-34-3View Details
Bis(pinacolato)diboron 98% CAS 73183-34-3View Details
73183-34-3 -
 Bis(pinacolato)diboron CAS 73183-34-3View Details
Bis(pinacolato)diboron CAS 73183-34-3View Details
73183-34-3
