BISMUTH TELLURIDE
Synonym(s):Bismuth sesquitelluride;Bismuth telluride;Dibismuth tritelluride
- CAS NO.:1304-82-1
- Empirical Formula: Bi2Te3
- Molecular Weight: 800.76
- MDL number: MFCD00014201
- EINECS: 215-135-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
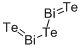
What is BISMUTH TELLURIDE?
Description
Bismuth telluride is a gray crystalline solid.Molecular weight=800.83; Specific gravity (H2O:1)=7.7;Freezing/Melting point=573℃; Vapor pressure=0 mmHgat 20℃. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 2, Reactivity 2.Note: Commercial mix of doped bismuth tritelluride maycontain 80% Bi2Te3, 20% stannous telluride, plus some tellurium. Insoluble in water. Doped and undoped have similarphysical properties.
Chemical properties
Bismuth telluride is a gray or black crystalline solid or gray powder.
The Uses of BISMUTH TELLURIDE
Bi2Te3, along with Sb2Te3 and other structurally analogous semiconductors, is widely regarded as one of the best materials for room-temperature thermoelectric devices. Bismuth(III) telluride is a key component of thermoelectric materials that have reached ZT values as high as 2.4 at room temperature.
The Uses of BISMUTH TELLURIDE
In electronics as semiconductor.
The Uses of BISMUTH TELLURIDE
Semiconductors; thermoelectric cooling; power generation application; for commercial use, Bi2Te3 is “doped” with selenium sulfide to alter its conductivity.
General Description
Gray or black hexagonal platelets with a metallic luster or gray powder. Mp: 586°C. Density: 7.8 g cm-3. An alloy of two metallic elements.
Reactivity Profile
BISMUTH TELLURIDE may emit toxic fumes of tellurium when heated to decomposition. Incompatible with strong oxidizing agents such as nitric acid or bromine, chlorine or fluorine. Moderate fire hazard by reaction with such materials. May react with strong acids to evolve toxic gases; may react slowly with water to evolve toxic gases. Slight explosion hazard with moisture.
Hazard
Toxic.
Health Hazard
Bismuth telluride, either alone
or doped with selenium sulfide, is apparently of
very low toxicity.
In limited industrial experimental work
with bismuth telluride under controlled conditions
(vacuum hoods), no adverse health
effects were encountered other than tellurium
breath.
Potential Exposure
Bismuth telluride is used in thermoelectric cooling, power generation, semiconductor manufacture; pharmaceuticals; medical therapy; cosmetics. Exposure involves those working in “Silicon Valley” and similar locations around the world. An alloy of two metallic elements that are sometimes doped with SeS. The SeS dopant alters the electrical properties, but has too low a concentration to affect the chemical properties.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make anunconscious person vomit.
Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, dimercaprol, dithiopropanol (C3H8OS2)] hasbeen used to treat toxic symptoms of certain heavy metalspoisoning. In the case of bismuth poisoning it may haveSOME value. Although BAL is reported to have a largemargin of safety, caution must be exercised, because toxiceffects may be caused by excessive dosage. Most can beprevented by premedication with 1-ephedrine sulfate(CAS: 134-72-5).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Bismuth telluride must bestored to avoid contact with strong oxidizers (such as chlorine, bromine, and fluoride), since violent reactions occur.Store in tightly closed containers in a cool, well-ventilatedarea away from moisture.
Shipping
UN3284 Tellurium compound, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Incompatibilities
Reacts with acids forming toxic gases. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Toxic and flammable gas may slowly evolve from contact with moisture.
Properties of BISMUTH TELLURIDE
| Melting point: | 585 °C(lit.) |
| Boiling point: | 1172 °C |
| Density | 7.642 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O; soluble in ethanol |
| form | Powder |
| color | Gray |
| Water Solubility | Insoluble in water. |
| Sensitive | Moisture Sensitive |
| Crystal Structure | Cubic, Halite Structure - Space Group Fm3m |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 5 mg/m3; TWA 0.1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; TWA 10 mg/m3; TWA 0.1 mg/m3 |
| CAS DataBase Reference | 1304-82-1(CAS DataBase Reference) |
| EPA Substance Registry System | Dibismuth tritelluride (1304-82-1) |
Safety information for BISMUTH TELLURIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BISMUTH TELLURIDE
| InChIKey | GUYIRKJSQUOSJV-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
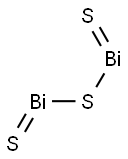
You may like
-
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride, - 350 mesh, 99.99% CAS 1304-82-1View Details
Bismuth(III) telluride, - 350 mesh, 99.99% CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1 -
 Bismuth(III) telluride CAS 1304-82-1View Details
Bismuth(III) telluride CAS 1304-82-1View Details
1304-82-1
