Bis(2-chloroethoxy)methane
- CAS NO.:111-91-1
- Empirical Formula: C5H10Cl2O2
- Molecular Weight: 173.04
- MDL number: MFCD00045293
- EINECS: 203-920-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02

What is Bis(2-chloroethoxy)methane?
Description
Bis(2-chloroethoxy)methane is a colorlessliquid. Molecular weight=173.1; Boiling point=218℃;Freezing/Melting point=233℃; Flash point=110℃(oc). Hazard Identification (based on NFPA-704 M RatingSystem): Health 2, Flammability 1, Reactivity 0. Slight solubility in water; solubility=0.8%.
Chemical properties
Bis(2-chloroethoxy)methane is a colorless liquid.
The Uses of Bis(2-chloroethoxy)methane
Bis(2-chloroethoxy)methane is an chloroether used to manufacture polysulfide polymers.
The Uses of Bis(2-chloroethoxy)methane
Manufacture of insecticides, polymers; degreasing solvent; intermediate for polysulfide rubber.
General Description
A colorless liquid. Boiling point 217.5°C, Flash point 230°F. Density 1.23 g / cm3. May be toxic by ingestion or inhalation. Severely irritates skin, eyes, and mucous membranes. Used as a solvent.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Bis(2-chloroethoxy)methane, the b-chloroethyl acetal of formaldehyde, is incompatible with oxidizing agents.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic by inhalation and ingestion; Strong irritant.
Fire Hazard
Not Flammable.
Potential Exposure
The chloroalkyl ethers have a wide variety of industrial uses in organic synthesis, treatment of textiles; the manufacture of polymers; polysulfide rubbers, and insecticides; as degreasing agents and solvents; and in the preparation of ion exchange resins.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Biological. Using settled domestic wastewater inoculum, bis(2-chloroethoxy)methane (5 and 10
mg/L) did not degrade after 28 d of incubation at 25 °C (Tabak et al., 1981).
Chemical/Physical. At influent concentrations of 10, 1.0, 0.1, and 0.01 mg/L, the GAC
adsorption capacities were 50, 11, 2.6, and 0.6 mg/g, respectively (Dobbs and Cohen, 1980).
storage
Color Code—Green: General storage may be used.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could createa potential fire or explosion hazard.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Incompatibilities
The aqueous solution is a strong acid; keep away from bases and alkaline material. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with mineral acids causes decomposition.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Destroy by high-temperature incineration with HCl scrubber.
Properties of Bis(2-chloroethoxy)methane
| Melting point: | -32.3°C |
| Boiling point: | 112°C/20mm |
| Density | 1.23 |
| vapor pressure | 1 at 53 °C (Weast, 1986) |
| refractive index | 1.45 |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Oil |
| color | Colorless liquid |
| Water Solubility | 81,000 mg/L at 25 °C using method of Moriguchi (1975) |
| Henry's Law Constant | (x 10-7 atm?m3/mol):
3.78 (calculated, U.S. EPA, 1980a) |
| CAS DataBase Reference | 111-91-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, bis(2-chloroethoxy)-(111-91-1) |
| EPA Substance Registry System | Bis(2-chloroethoxy)methane (111-91-1) |
Safety information for Bis(2-chloroethoxy)methane
Computed Descriptors for Bis(2-chloroethoxy)methane
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
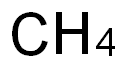




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1