Bilirubin
Synonym(s):Bile pigment;Bilirubin - CAS 635-65-4 - Calbiochem;Hemetoidin
- CAS NO.:635-65-4
- Empirical Formula: C33H36N4O6
- Molecular Weight: 584.66
- MDL number: MFCD00005499
- EINECS: 211-239-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-28 10:40:26
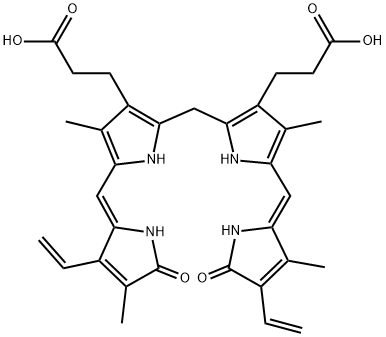
What is Bilirubin?
Description
Bilirubin is a yellow breakdown product of heme catabolism, formed when heme is cleaved by heme oxygenase. This reaction produces carbon monoxide and biliverdin, which is rapidly reduced to bilirubin by biliverdin reductase. Bilirubin has key roles as a free radical scavenger with antioxidant and anti-inflammatory actions. Free and albumin-bound bilirubin can also scavenge nitric oxide (NO) and NO-related species. Unconjugated bilirubin is highly water-insoluble and must be conjugated with glucuronides to become water soluble and subsequently excreted by the liver and kidney.
Description
The bile pigment bilirubin is the major end product of the breakdown of heme in the liver. It is excreted in bile and urine. Its yellow color is responsible for the yellowish appearance of patients with jaundice.
In 1847, German scientist Rudolf Virchow and his colleagues isolated bilirubin crystals from hematomas and conjectured that it was derived from blood. More than a century later, Irving M. London and co-workers at Columbia University demonstrated that heme is indeed its source.
The central structure of heme is a porphyrin ring. Bilirubin’s structure is that of a ring-opened porphyrin: a chain of substituted pyrrole and 1,3-dihydro-2H-pyrrol-2-one rings.
The determination of bilirubin concentrations in blood is a key test for diagnosing medical conditions such as hepatobiliary or heart disease. Traditionally, blood tests have been performed in laboratories and can take several hours to complete. Patient-operated finger-stick tests with appropriate analytical equipment theoretically would take less time and be less expensive. But the recent Theranos debacle showed that?this technology is not ready for prime time. Improvements in biosensors, sampling methods, and other factors are necessary to make this method practical.
Chemical properties
Light Orange to Deep Redish-Brown Crystalline Solid
The Uses of Bilirubin
Bilirubin is widely utilized to monitor the liver function and their disease, such as hepatitis or cirrhosis; or the effects of medicines which are damage the liver. It acts as a pigment in certain algae to capture light energy. Its double bonds are isomerizes in the presence of light, which is employed in phototherapy of jaundiced newborns babies. It is used to detect the blocking in bile ducts.
The Uses of Bilirubin
Principal pigment of bile and constituent of many biliary calculi
The Uses of Bilirubin
Bilirubin has been used:
- in phantom preparation
- in in vitro experiments
- in the preparation of bilirubin solutions for infusion
What are the applications of Application
Bilirubin is a principal pigment of bile and one of the major end products of hemoglobin decomposition
Definition
Red coloring matter of bile. Also occurs in blood serum as decomposition product of hemoglobin.
General Description
Bilirubin, a heme catabolism end-product is produced by the reduction of its metabolic precursor biliverdin by the action of enzyme biliverdin reductase.
Biochem/physiol Actions
Bilirubin is an active oxidative DNA cleaving agent as well as an effective antioxidant agent, a hydroxyl radical quencher. This bile pigment has both antioxidant and toxic properties. It is a natural inhibitor of vascular smooth muscle cell proliferation (VSMCs). It displays anti-inflammatory and anti-proliferative properties when used as a therapeutic agent in lung/vascular diseases. Serum bilirubin concentration slightly above the normal levels have shown a lesser incidence of heart disease. It is a potential therapeutic agent in heart transplantation and T-cell mediated immune disorders.
Purification Methods
An acyclic tetrapyrrole bile pigment with impurities which can be eliminated by successive Soxhlet extraction with diethyl ether and MeOH. It crystallises from CHCl3 as deep red-brown rhombs, plates or orange-red prisms from chlorobenzene (m 330o dec) and is dried to constant weight at 80o under vacuum. [Gray et al. J Chem Soc 2264, 2276 1961, Beilstein 26 III/IV 3268.]
Properties of Bilirubin
| Melting point: | 192 °C |
| Boiling point: | 641.7°C (rough estimate) |
| Density | 1.2163 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | -20°C |
| solubility | aqueous acid: soluble |
| form | powder |
| pka | 4.62±0.10(Predicted) |
| color | orange |
| Water Solubility | practically insoluble |
| Sensitive | Light Sensitive |
| Merck | 14,1218 |
| BRN | 74376 |
| Stability: | Stable. Refrigerate. |
| EPA Substance Registry System | Bilirubin (635-65-4) |
Safety information for Bilirubin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bilirubin
Bilirubin manufacturer
Dr. Silviu Pharmachem Pvt., Ltd.
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Bilirubin, GR 99%+ CAS 635-65-4View Details
Bilirubin, GR 99%+ CAS 635-65-4View Details
635-65-4 -
 Bilirubin extrapure AR CAS 635-65-4View Details
Bilirubin extrapure AR CAS 635-65-4View Details
635-65-4 -
 Bilirubin 95% CAS 635-65-4View Details
Bilirubin 95% CAS 635-65-4View Details
635-65-4 -
 Bilirubin CAS 635-65-4View Details
Bilirubin CAS 635-65-4View Details
635-65-4 -
 BILIRUBIN AR CAS 635-65-4View Details
BILIRUBIN AR CAS 635-65-4View Details
635-65-4 -
 Bilirubin CAS 635-65-4View Details
Bilirubin CAS 635-65-4View Details
635-65-4 -
 Bilirubin CAS 635-65-4View Details
Bilirubin CAS 635-65-4View Details
635-65-4 -
 Bilirubin CAS 635-65-4View Details
Bilirubin CAS 635-65-4View Details
635-65-4