BIBF-1120
- CAS NO.:928326-83-4
- Empirical Formula: C31H33N5O4
- Molecular Weight: 539.633
- MDL number: MFCD11974012
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-30 11:18:30
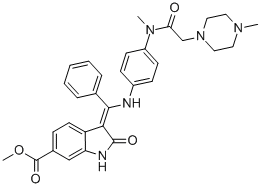
What is BIBF-1120?
Description
BIBF 1120 is a triple kinase inhibitor blocking vascular endothelial growth factor receptor, PDGF receptor, and FGF receptor, which has a potential to inhibit tumor growth and pulmonary fibrosis.
Characteristics
Primary targets: PDGFR; VEGFR; FGFR
Class: receptor tyrosine kinase
Treatment: ILD, IPF
Oral bioavailability = 4.7%
Elimination half-life = 10
Definition
ChEBI: Nintedanib is a member of the class of oxindoles that is a kinase inhibitor used (in the form of its ethylsulfonate salt) for the treatment of idiopathic pulmonary fibrosis and cancer. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor, a vascular endothelial growth factor receptor antagonist, a fibroblast growth factor receptor antagonist and an angiogenesis inhibitor. It is an aromatic ester, a methyl ester, a member of oxindoles, an enamine, an aromatic amine, an aromatic amide and a N-alkylpiperazine. It is a conjugate base of a nintedanib(1+).
brand name
OfevTM
Anticancer Research
BIBF 1120 is a potent inhibitor of VEGFR as well as PDGF and fibroblast growth factor receptor. In a randomized phase II placebo-controlled trial, patients who had just completed chemotherapy for relapsed ovarian cancer, with evidence of response, but at high risk of further early recurrence were treated with BIBF 1120. The study drug was taken continuously (28-day cycles) for 9 cycles (36 weeks) or until disease progression or patient withdrawal. The 36-week PFS 26-week rates were 16.3% and 5.0% in the BIBF 1120 and placebo groups, respectively (HR 0.65; 95% Cl, 0.42 to 1.02; P = .06). Toxicity was also well tolerated.This has prompted a phase III trial (NCTO1O15118) where BIBF 1120 will be combined with carboplatin/paclitaxel as front-line chemotherapy in ovarian cancer.
Side Effects
Most of the adverse effects associated with BTBF-1120 were tolerable. They include diarrhea, nausea, vomiting, abdominal pain, and reversible increase in liver enzymes.
Clinical claims and research
BIBF 1120 is a triple tyrosine kinase inhibitor with efficacy on fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and on platelet-derived growth factor (PDGF).The activation of these pathways has been implicated in the pathogenesis of experimental fibrosis. In a 12-month phase II trial (the TOMORROW trial) patients who received 150 mg of BIBF 1120 twice daily had a trend toward reduction in the decline of FVC.In addition, there was an improved QOL and a reduction in acute exacerbations of IPF. These results led to a phase III trial. This was designed as two identical phase III studies called INPULSIS-1 and INPULSIS-2. The INPULSIS trials tested the effect on IPF disease progression over 52 weeks using 150 mg of twice daily nintedanib (formerly BIBF 1200) versus placebo. The inclusion criteria included patients diagnosed with idiopathic pulmonary fibrosis based on established criteria.In addition, the participants’ HRCT scans and biopsies, if available, were reviewed by a central radiologist or pathologist to confirm the diagnosis. Enrolled subjects had a FVC which was >50% of the predicted value and a DlCO from 30–79% of predicted values. Published data from the two trials indicated that patients who received nintedanib demonstrated a statistically significant reduction in the rate of decline in lung function compared with the placebo group. In the INPULSIS-2 trial, there was a significant reduction in the time to first acute exacerbation of IPF. This was not replicated in the INPULSIS-1 trial data. The most common side effect for the medication was diarrhea. On the basis of this study, nintedanib is pending evaluation for approval for use in the United States.
Safety information for BIBF-1120
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![1,3-DiMethyl 2-[4-(Methoxycarbonyl)-2-nitrophenyl]propanedioate](https://img.chemicalbook.in/CAS/GIF/1160293-27-5.gif)


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
