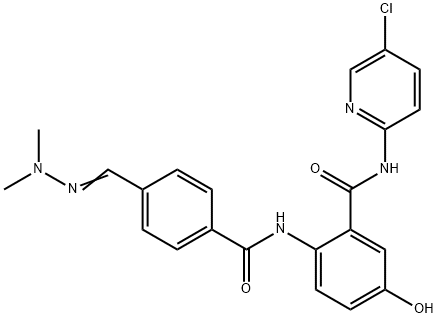
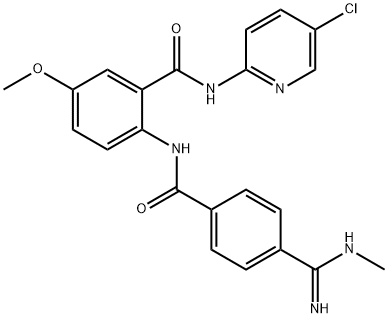
Betrixaban
Synonym(s):;N-(5-Chloro-2-pyridinyl)-2-[[4-[(dimethylamino)iminomethyl]benzoyl]amino]-5-methoxybenzamide;N-(5-Chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide;
- CAS NO.:330942-05-7
- Empirical Formula: C23H22ClN5O3
- Molecular Weight: 451.91
- MDL number: MFCD16038040
- EINECS: 682-459-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
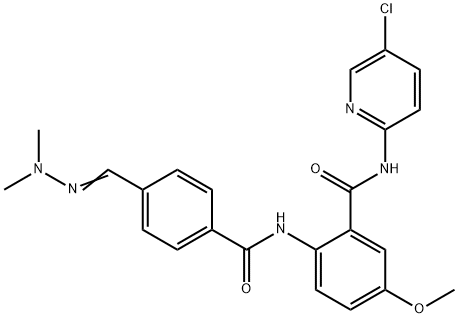
What is Betrixaban?
Absorption
Betrixaban presents a rapid absorption at a dose of 80 mg. Its peak plasma concentration is registered within 3-4 hours after oral administration in healthy humans. The oral bioavailability is 34%, and it can be reduced with the consumption of food. Specifically, the Cmax and AUC is reduced by an average of 70% and 61% with a low-fat meal, and 50% and 48% with a high-fat meal compared to the fasted state, an effect which is apparent up to six hours following food intake.
Toxicity
Betrixaban presents a minimal hepatotoxicity, which is the main adverse effect found in this class of drugs. Some of the major adverse effects of Betrixaban are bleeding or hypersensitivity .
The Uses of Betrixaban
Betrixaban is a novel anticoagulant, an oral direct inhibitor of factor Xa (FXa) for use in the prevention of venous thromboembolism (VTE).
Indications
Betrixaban is indicated for prophylaxis of venous thromboembolism (VTE) in conditions of moderate to severe restricted mobility or in patients that qualify as in risk of VTE.
Background
Betrixaban is a non-vitamin K oral anticoagulant whose action is driven by the competitive and reversible inhibition of the factor Xa . It was selected among all lead compounds due to its low hERG channel affinity while sustaining its factor Xa inhibition capacity . Betrixaban, now developed by Portola Pharmaceuticals Inc., is prescribed as a venous thromboembolism (VTE) prophylactic for adult patients with moderate to severe restricted motility or with other risks for VTE . VTE can be manifested as deep vein thrombosis or pulmonary embolism and it is a leading cause of preventable death in hospitalized patients .
Definition
ChEBI: Betrixaban is a secondary carboxamide obtained by formal condensation of the carboxy group of 4-(N,N-dimethylcarbamimidoyl)benzoic acid with the amino group of 2-amino-N-(5-chloropyridin-2-yl)-5-methoxybenzamide. A synthetic anticoagulant compound that targets activated factor Xa in the coagulation cascade. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a member of guanidines, a member of benzamides, a secondary carboxamide, a monochloropyridine and a monomethoxybenzene.
Biological Activity
betrixaban is a highly potent, selective, and orally efficacious inhibitor of factor xa with ic50 value of 1.5nm [1].betrixaban shows excellent anticoagulant potency in vitro. in the rabbit deep vein thrombosis model, the concentration of betrixaban required to double the rabbit prothrombin time is below 2μm. betrixaban is selective for fxa and has poor activity for thrombin, trypsin, t-pa and apc. the patch clamp herg assay shows that betrixaban has low affinity with herg suggesting it is safer than other candidate compounds. in addition, betrixaban displays a profile of good oral bioavailability and oral exposure, long half-life in animal models. it has bioavailability of 51.6% at dose of 0.5 mg/kg iv and 2.5 mg/kg po. furthermore, the phase ii study has proved betrixaban as an oral fxa inhibitor for prevention of venous thromboembolic events [1].
Pharmacokinetics
Betrixaban is an oral anticoagulant that excerts its action by preventing thrombin generation without having a direct effect on platelet aggregation .
Metabolism
One of the major characteristics of Betrixaban is its minimal hepatic metabolism (< 1%), preventing potential accumulation with liver impariment. Unchanged Betrixaban is the main form found in human plasma, followed by two hydolitic CYP-independent inactive metabolites (15-18%). The minimal hepatic metabolism produces an unlikely drug-to-drug interaction with inhibitors or agonists of CYP450 .
References
[1] zhang p, huang w, wang l, bao l, jia zj, bauer sm, goldman ea, probst gd, song y, su t, fan j, wu y, li w, woolfrey j, sinha u, wong pw, edwards st, arfsten ae, clizbe la, kanter j, pandey a, park g, hutchaleelaha a, lambing jl, hollenbach sj, scarborough rm, zhu by. discovery of betrixaban (prt054021), n-(5-chloropyridin-2-yl)-2-(4-(n,n-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor xa inhibitor. bioorg med chem lett. 2009 apr 15;19(8):2179-85.
Properties of Betrixaban
| Melting point: | 210-213°C |
| Density | 1.30±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.00±0.70(Predicted) |
| color | Off-White |
| CAS DataBase Reference | 330942-05-7 |
Safety information for Betrixaban
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Betrixaban
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![N-(5-Chloro-2-pyridinyl)-2-[[4-[(dimethylamino)iminomethyl]benzoyl]amino]-5-methoxybenzamide (2Z)-2-butenedioate (1:1)](https://img.chemicalbook.in/CAS/20180808/GIF/936539-80-9.gif)
You may like
-
 Betrixaban CAS 330942-05-7View Details
Betrixaban CAS 330942-05-7View Details
330942-05-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
