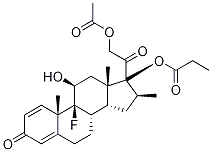
Betamethasone 17-valerate
Synonym(s):1,4-Pregnadiene-11β,17α,21-triol-9α-fluoro-16β-methyl-3,20-dione 17-valerate;9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 17-valerate;9α-Fluoro-16β-methylprednisolone 17-valerate;Betamethasone 17-valerate;Betnovate
- CAS NO.:2152-44-5
- Empirical Formula: C27H37FO6
- Molecular Weight: 476.58
- MDL number: MFCD00867446
- EINECS: 218-439-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
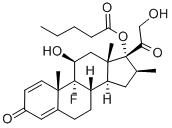
What is Betamethasone 17-valerate?
Description
Betamethasone 17-valerate is a synthetic glucocorticoid ester. It is the 17-valerate ester of betamethasone. Betamethasone 17-valerate is often used to treat mild eczema with good efficacy and lower incidence of steroid induced adverse effects due to its lower potency compared to other glucocorticoids. It is available in cream, ointment, lotion, and foam preparations for topical use.
Chemical properties
White Solid
Originator
Valisone,Schering,US,1967
The Uses of Betamethasone 17-valerate
Betamethasone 17-valerate is a potent glucocorticoid steroid with anti-inflammatory and immunosuppressive properties. Topical application of Betamethasone 17-Valerate has been shown to inhibit heat-induced vasodilatation in man.
Definition
ChEBI: Betamethasone valerate is a steroid ester that is betamethasone in which the hydroxy group at the 17alpha position has been converted to the corresponding pentanoate ester. It has a role as an anti-inflammatory drug. It is a steroid ester, a 20-oxo steroid, a 21-hydroxy steroid, an 11beta-hydroxy steroid, a fluorinated steroid, a 3-oxo-Delta(1),Delta(4)-steroid and a primary alpha-hydroxy ketone. It is functionally related to a betamethasone.
Indications
Betamethasone valerate (Psorion, Beta-Val, Luxiq) is a synthetic fluorinated corticosteroid.
Manufacturing Process
The Betamethasone 17-valerate is made from betamethasone as a starting material as follows: A suspension of 9α-fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene- 3,20-dione(betamethasone) (2 grams) in sodium dried benzene (500 ml) was distilled vigorously for a few minutes, toluene-p-sulfonic acid monohydrate (30 mg) and methyl orthovalerate (5 ml) were added and distillation was continued for 10 minutes. The mixture was then boiled under reflux for 1.5 hours after which time unreacted betamethasone alcohol (400 mg) was removed by filtration. The benzene solution was treated with solid sodium .bicarbonate and a few drops of pyridine, filtered and evaporated to dryness at about 50°C. The residue, in ether, was filtered through grade III basic alumina (20grams) to remove traces of unreacted betamethasone alcohol, the ether removed in vacuo and the residue of crude betamethasone 17,21- methyl orthovalerate was treated with acetic acid (20ml) and a few drops of water and left overnight at room temperature.
The acetic acid solution was poured into water (100 ml) and extracted with chloroform. The chloroform extracts were washed in turn with water, saturated sodium bicarbonate solution and water, dried and evaporated in vacuo. The residual gum was triturated with ether and a white crystalline solid (1.16 grams) isolated by filtration. Recrystallization from ether (containing a small amount of acetone)-petroleum ether gave 9α-fluoro-11β,21-dihydroxy-16β- methyl-17α-valeryloxypregna-1,4-diene-3,20-dione (871 mg) as fine needles.
brand name
Betatrex (Savage); Luxiq (Connetics); Valisone (Schering).
Therapeutic Function
Corticosteroid
Biochem/physiol Actions
Betamethasone 17-valerate is a synthetic glucocorticoid. It exhibitstherapeutic effects against various allergic and inflammatory skin diseases. Betamethasone 17-valerate also possesses anti-inflammatory properties.
Clinic research
Betamethasone 17-valerate, a new corticosteroid synthesized expressly for topical use, was clinically evaluated in treating various dermatoses in a two-part study. In the first part, a clinical-use study, betamethasone 17-valerate 0.1% cream produced excellent to good results in 139 (84%) of 166 patients. In the second part, a double-blind, bilateral, symmetrical paired comparison, betamethasone 17-valerate 0.05% ointment proved superior to the other corticosteroid in 37 (69%) of 54 patients, and the two were equally efficacious in 16 (29%). With the exception of two patients who developed local irritation, no gross evidence of systemic absorption or other adverse effects was noted.
Properties of Betamethasone 17-valerate
| Melting point: | 183-184° |
| Boiling point: | 598.9±50.0 °C(Predicted) |
| alpha | D +77° (dioxane) |
| Density | 1.1174 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in acetone and in methylene chloride, soluble in ethanol (96 per cent). |
| form | neat |
| pka | 12.90±0.70(Predicted) |
| form | Solid |
| color | White |
| Merck | 13,1183 |
| BRN | 4240001 |
| CAS DataBase Reference | 2152-44-5(CAS DataBase Reference) |
| EPA Substance Registry System | .beta.-Methasone 17-valerate (2152-44-5) |
Safety information for Betamethasone 17-valerate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Betamethasone 17-valerate
| InChIKey | SNHRLVCMMWUAJD-SUYDQAKGSA-N |
| SMILES | [C@]12(C)C[C@@H]([C@]3(F)[C@@]4(C)C=CC(=O)C=C4CC[C@@]3([H])[C@]1([H])C[C@H](C)[C@@]2(C(=O)CO)OC(=O)CCCC)O |&1:0,3,4,6,16,18,21,23,r| |
Betamethasone 17-valerate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Betamethasone Valerate 99%View Details
Betamethasone Valerate 99%View Details -
 Betamethasone valerate 99%View Details
Betamethasone valerate 99%View Details -
 2152-44-5 99%View Details
2152-44-5 99%View Details
2152-44-5 -
 Betamethasone valerate CAS 2152-44-5View Details
Betamethasone valerate CAS 2152-44-5View Details
2152-44-5 -
 Betamethasone Valerate Api, BPView Details
Betamethasone Valerate Api, BPView Details
2152-44-5 -
 Betamethasone Valerate Usp 2152-44-5 API Bulk Drugs, PowderView Details
Betamethasone Valerate Usp 2152-44-5 API Bulk Drugs, PowderView Details
2152-44-5 -
 Betamethasone Valerate Cas No 2152 44 5, Packaging Size: 10 gmView Details
Betamethasone Valerate Cas No 2152 44 5, Packaging Size: 10 gmView Details
2152-44-5 -
 Betamethasone 17 Valerate CAS Number: 2152-44-5View Details
Betamethasone 17 Valerate CAS Number: 2152-44-5View Details
2152-44-5
