bestrabucil
- CAS NO.:75219-46-4
- Empirical Formula: C41H47Cl2NO6
- Molecular Weight: 720.729
- MDL number: MFCD00866413
- Update Date: 2024-10-23 13:36:13
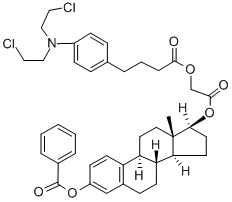
What is bestrabucil?
Originator
Atrimustine,Onbio Inc.
Manufacturing Process
Preparation of 3-hydroxy-1,3,5(10)-estratriene-17β-[4-{p-[bis(2-
chloroethyl)amino]phenyl}butyryloxy]acetate:
Preparation of 3-hydroxy-1,3,5(10)-estratriene-17β-monobromoacetate. 10 g
of 1,3,5(10)-estratriene-3,17β-diol was dissolved in 400 ml of anhydrous
tetrahydrofuran (THF), and then, 8.8 g of pyridine was added. A solution of
22.5 g of monobromoacetyl bromide in 74 g of carbon tetrachloride was
added dropwise to the resulting solution at about -5°C to -7°C. The mixture
was kept for one night. After the reaction, the resulting precipitate was
separated by a filtration. The solvent was distilled off from the filtrate. The
residue was dissolved in ether and recrystallized from ether to obtain
1,3,5(10)-estratriene-3,17β-bis(monobromoacetate). 2 g of the product was
dissolved in 900 ml of methanol and the solution was cooled to -5°C. A
solution of 0.24 g of K2CO3 in 20 ml of water was added dropwise to the
resulting solution. After the reaction for 30 minutes, 1000 ml of water was
added and the resulting precipitate was separated and dried. It was confirmed
that the product was 3-hydroxy-1,3,5(10)-estradiene-17β-monobromoacetate
by the elementary analysis and the IR spectrum.
Preparation of 3-hydroxy-1,3,5(10)-estratriene-17β-[4{p-[bis(2-
chloroethyl)amino]phenyl}butyryloxy]acetate:
200 mg of silver [4-{p-[bis(2-chloroethyl)amino]phenyl}butyrate (silver salt
of Chlorambucil) was added in 10 ml of DMSO to form a white colloidal
solution. Then, 190.8 mg of 3-hydroxy-1,3,5(10)-estratriene-17β-
monobromoacetate was added and the mixture was stirred at room
temperature for 64 hours in the dark. The precipitate was changed to
yellowish green color. A small amount of acetone was added and the
precipitate was separated by a filtration through G-4 filter. The precipitate was
changed from yellowish green color to blackish green color by the irradiation
of light. The filtrate was colorless and transparent. DMSO was distilled off
under a reduced pressure on a water bath at 80°C and 100 ml of water was
added to precipitate white crystals. The crystals were kept for 1 hour to
remove DMSO and the crystals were separated through G-4 filter and
thoroughly washed with distilled water and dried under a reduced pressure in
a desiccator. A crude yield was 330.5 mg.
Purification of the product 330.5 mg of crude crystals were dissolved in a
mixed solvent of 50 vol. parts of cyclohexane and 10 vol. parts of ethyl
acetate. The solution was slowly passed through a column filling 40 g of silica
gel and the product was gradually separated to obtain 188.2 mg (yield:
62.86%) of pure product. It was confirmed that the product was 3-hydroxy-1,3,5(10)-estratriene-17β-[4{p-[bis(2-chloroethyl)amino]phenyl}
butyryloxy]acetate by the elementary analysis and the IR spectrum.
Therapeutic Function
Antineoplastic
Safety Profile
An experimental teratogen. Otherexperimental reproductive effects. Human mutation datareported. When heated to decomposition it emits toxicfumes of Cl- and NOx.
Properties of bestrabucil
| Boiling point: | 804.3±65.0 °C(Predicted) |
| Density | 1.1424 (rough estimate) |
| refractive index | 1.7350 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Solid |
| pka | 4.65±0.50(Predicted) |
| color | Crystals |
Safety information for bestrabucil
Computed Descriptors for bestrabucil
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1