Benznidazole
- CAS NO.:22994-85-0
- Empirical Formula: C12H12N4O3
- Molecular Weight: 260.25
- MDL number: MFCD00243089
- EINECS: 200-659-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
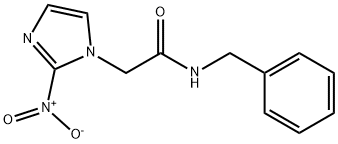
What is Benznidazole?
Absorption
Benznidazole has a bioavailability of 91.7% and a Tmax of 2.93 h .
Toxicity
At clinically relevant dosages, benznidazole can produce hepatotoxicity, peripheral neuropathy, and angioedema .
Description
Benznidazole is the second of the drugs approved for treatment of Chagas' disease. Like nifurtimox, it is effective against the circulating form of Trypanosoma cruzi during the acute phase of the disease, but also like nifurtimox, it is ineffective during the chronic stage of the disease.
Description
Benznidazole is an orally bioavailable antiprotozoal agent. It is a 2-nitroimidazole prodrug that becomes active when the nitro group is reduced within the parasite. It inhibits the growth of the parasites T. cruzi, T. vaginalis, G. lamblia, and E. histolytica (IC50s = 8.1, 18.62, 22.58, and 4.27 μM, respectively). It also inhibits clonogenic growth of human C33A cervical and KNS42 glioblastoma cancer cells under hypoxic, but not normoxic, conditions when used at a concentration of 100 μM. Benznidazole (100 mg/kg per day) decreases T. cruzi blood parasitemia to below detectable levels in a mouse model of chronic stable Chagas disease. Formulations containing benznidazole have been used in the treatment of Chagas disease caused by T. cruzi.
The Uses of Benznidazole
Benznidazole (BNZ) is traditionally used to treat Chagas disease caused by Trypanosoma cruzi. The drugs used for the treatment of this disease, Nifurtimox and Benznidazole, are toxic and present sever e side effects.
The Uses of Benznidazole
N-Benzyl-2-nitro-1H-imidazole-1-acetamide (Benznidazole) may be used as reference drug for the extraction of guaianolide from the aerial parts of Tanacetum parthenium and to evaluate its in vitro antiprotozoal activity.
Indications
For use in the treatment of Chagas disease in children 2-12 years of age .
Background
Benznidazole was granted accelerated approval for the treatment of Chagas disease in children 2-12 years of age by the FDA on August 29, 2017. It is the first treatment made available in the United States for Chagas disease.
Definition
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2-nitroimidazol-1-yl)acetic acid with the aromatic amino group of benzylamine. Used for treatment of Chagas disease.
Antimicrobial activity
It exhibits antiprotozoal activity, particularly against Trypanosoma cruzi.
General Description
N-Benzyl-2-nitro-1H-imidazole-1-acetamide (Benznidazole) is a nitro-heterocyclic compound. It is widely employed drug for the treatment of Chagas disease. It exhibits three polymorphic forms..
Pharmaceutical Applications
A synthetic 2-nitroimidazole, formulated for oral administration. Solubility in water 400 mg/L.
Mechanism of action
Studies suggest that benznidazole does not catalyze the formation of ROS and, therefore, has a mechanism of action different from that of nifurtimox. It has been proposed that benznidazole undergoes an one-electron transfer to the nitro group, which in turn dismutates to give back the nitroimidazole and a nitrosoimidazole. The latter product may then undergo an electrophilic addition to trypanothione, which leads to depletion of trypanothione, an essential enzyme system in the Trypanosoma cruzi.
Pharmacokinetics
Benznidazole is a trypanocidal agent which kills the causative organism in Chagas disease, Trypanosoma cruzi .
Pharmacokinetics
Oral bioavailability :High
Cmax 100 mg oral :2.2–2.8 mg/L after 3–4 h
Plasma half-life:10.5–13.6 h
Volume of distribution:c. 0.56 L/kg
Plasma protein binding: c. 44%
The 2-nitro group undergoes reduction to the amine and hydrolysis to the hydroxy derivative.
Clinical Use
Benznidazole is used in treatment of South American trypanosomiasis (Chagas disease).
Clinical Use
N-Benzyl-2-nitroimidazole-1-acetamide (Radanil, Rochagan)is a nitroimidazole derivative that is used for the treatment ofChagas disease. It is not available in the United States but isused extensively in South America. The effectiveness of benznidazoleis similar to that of nifurtimox. Therapy forAmerican trypanosomiasis with oral benznidazole requiresseveral weeks and is frequently accompanied by adverse effectssuch as peripheral neuropathy, bone marrow depression,and allergic-type reactions.
Side Effects
Adverse effects are more common in the elderly and include nausea, vomiting, abdominal pain, peripheral neuropathy and severe skin reactions.
Metabolism
Benznidazole is metabolized by nitroreductases in Trypanosoma cruzi and by cytochrome P450 enzymes .
Properties of Benznidazole
| Melting point: | 189-192 °C (lit.) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | methanol: soluble50mg/mL, clear, colorless to yellow |
| form | Solid |
| pka | 14?+-.0.46(Predicted) |
| color | White to Off-White |
| Water Solubility | 0.4g/L(temperature not stated) |
| Merck | 13,1085 |
Safety information for Benznidazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benznidazole
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran




You may like
-
 N-Benzyl-2-nitro-1H-imidazole-1-acetamide CAS 22994-85-0View Details
N-Benzyl-2-nitro-1H-imidazole-1-acetamide CAS 22994-85-0View Details
22994-85-0 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3