Tinidazole
Synonym(s):1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole;Tinidazole
- CAS NO.:19387-91-8
- Empirical Formula: C8H13N3O4S
- Molecular Weight: 247.27
- MDL number: MFCD00057217
- EINECS: 243-014-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34

What is Tinidazole?
Absorption
Rapidly and completely absorbed under fasting conditions. Administration with food results in a delay in Tmax of approximately 2 hours and a decline in Cmax of approximately 10% and an AUC of 901.6 ± 126.5 mcg hr/mL.
Toxicity
There are no reported overdoses with tinidazole in humans. In acute studies with mice and rats, the LD 50 for mice was generally > 3,600 mg/kg for oral administration and was > 2,300 mg/kg for intraperitoneal administration. In rats, the LD 50 was > 2,000 mg/kg for both oral and intraperitoneal administration.
Chemical properties
solid
Originator
Simplotan,Pfizer,W. Germany,1971
The Uses of Tinidazole
Antiprotozoal (Trichomonas, Giardia); antiamebic; antibacterial.
The Uses of Tinidazole
anticonvulsant
The Uses of Tinidazole
anti-ulcerative
The Uses of Tinidazole
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestin
Background
A nitroimidazole antitrichomonal agent effective against Trichomonas vaginalis, Entamoeba histolytica, and Giardia lamblia infections.
Indications
For the treatment of trichomoniasis caused by T. vaginalis in both female and male patients. Also for the treatment of giardiasis caused by G. duodenalis in both adults and pediatric patients older than three years of age and for the treatment of intestinal amebiasis and amebic liver abscess caused by E. histolytica in both adults and pediatric patients older than three years of age.
What are the applications of Application
Tinidazole is a synthetic imidazole antiprotozoal agent
Definition
ChEBI: Tinidazole is 1H-imidazole substituted at C-1 by a (2-ethylsulfonyl)ethyl group, at C-2 by a methyl group and at C-5 by a nitro group. It is used as an antiprotozoal, antibacterial agent. It has a role as an antiprotozoal drug, an antibacterial drug, an antiparasitic agent and an antiamoebic agent.
Manufacturing Process
The preparation of ethylsulfonylethyl-p-toluenesulfonate is carried out in the
following manner: 69.0 grams (0.5 mol) ethylsulfonylethanol dissolved in 150
ml pyridine is cooled to 0°C with stirring and while maintaining the
temperature between 0° to 10°C, 95 grams (0.5 mol) p-toluenesulfonyl
chloride is added in portions over a 10 minute period. After this time, 250 ml
water is added slowly and the mixture extracted with chloroform, the organic
phase washed first with 2 N HCl, then with water, separated and dried. The
product which crystallizes on cooling is filtered and dried to give 77.5% yield
of this intermediate.
A mixture of 12.7 grams (0.1 mol) of 2-methyl-5-nitroimidazole and 58.4
grams (0.2 mol) ethylsulfonylethyl-p-toluenesulfonate is heated with stirring,
under nitrogen, at 145° to 150°C for about 4 hours. After this time, the
reaction mixture is extracted with 500 ml hot water, the aqueous portion
adjusted with 10% Na2CO3 to a pH of 9 and extracted with chloroform (3
times with 150 ml portions). The separated organic phase is washed with
water, dried with Na2SO4 and evaporated to dryness. The crude tinidazole
product is then crystallized from benzene to give 4.36 grams of product
having a MP of 127° to 128°C.
Therapeutic Function
1-[2-(Ethylsulfonyl)ethyl]-2-methyl-5-nitroimidazole
Antimicrobial activity
Its antibacterial and antiprotozoal activity is similar to that of metronidazole.The MIC against G. vaginalis is 0.2–2 mg/L; the hydroxy metabolite is significantly more active than that of metronidazole. H. pylori is inhibited by 0.5 mg/L. T. vaginalis and T. fetus at 2.5 mg/L and E. histolytica is inhibited by about 0.3–2.5 mg/L.
Pharmaceutical Applications
A 5-nitroimidazole available for oral administration and, in some countries, for intravenous infusion.
Mechanism of action
Tinidazole has a mechanism of action that parallels that of metronidazole as well as a similar metabolic pathway leading to hydroxylation at the 2-methyl group catalyzed by CYP3A4. Basically, tinidazole appears to mimic the actions of metronidazole, although there are reports that it is effective against some protozoa which are resistant to metronidazole.
Pharmacokinetics
Tinidazole is a synthetic antiprotozoal agent. Tinidazole demonstrates activity both in vitro and in clinical infections against the following protozoa: Trichomonas vaginalis, Giardia duodenalis (also termed G. lamblia), and Entamoeba histolytica. Tinidazole does not appear to have activity against most strains of vaginal lactobacilli.
Pharmacokinetics
Oral absorption :>95%
Cmax 2 g oral:40 mg/L after 2 h
800 mg (30-min infusion): 12 mg/L 6 min after end infusion
Plasma half-life: 12–14 h
Volume of distribution 0.64 L/kg :Plasma protein binding 12%
absorption and distribution
After a 2 g oral dose, concentrations remain at c. 10 mg/L at 24 h and 2.5 mg/L at 48 h. Daily doses of 1 g maintain plasma levels in excess of 8 mg/L, irrespective of whether the dose is oral or intravenous. It is well distributed, with concentrations in bile, CSF, breast milk and saliva similar to those reached in plasma.
The drug readily crosses the placenta. In women undergoing first trimester abortion, concentrations of 4.9 mg/kg (placenta) and 7.6 mg/kg (fetus) were found when the plasma concentration was 13.2 mg/L.
Metabolism and excretion
Metabolites include the 2-hydroxymethyl derivative, its glucuronide and two unidentified minor derivatives. In urine about half the drug remains unmetabolized.
The parent drug and its metabolites are excreted primarily in the urine and to a minor extent in the feces. The clearance rate is about 0.73 mL/min per kg and the urinary excretion is about 21% of the dose. Total clearance of the drug is 51 mL/min, renal clearance 10 mL/min. In healthy volunteers given an : intravenousinfusion of 800 mg [14C]tinidazole over 30 min, a mean of 44% of the dose was excreted in the urine during the first 24 h, increasing to 63% over 5 days: only 12% of the dose appeared in the feces. Unchanged tinidazole comprised 32% of urinary 14C in 0&ndash:12 h urine. The 2-hydroxymethyl metabolite accounted for about 9% of the urinary 14C and was also present in plasma.
In renal failure the pharmacokinetics are not significantly different from those in healthy individuals. It is rapidly removed by hemodialysis and a normal dose should be given after each dialysis: if treatment precedes dialysis a half dose should be infused after the end of the procedure.
Clinical Use
Anaerobic bacterial infections(prophylaxis and treatment)
Trichomoniasis
Giardiasis (single dose) Amebiasis (including amebic liver abscess)
Bacterial vaginosis
Gastric colonization with H. pylori (in combination with other agents)
Side Effects
Tinidazole is generally well tolerated. Infrequent and transient effects include nausea, vomiting, diarrhea and a metallic taste. Disulfiram-like reactions may occur and rare neurological disturbances and transient leukopenia have been described. Rash, which may be severe, urticaria and angioneurotic edema can occur.
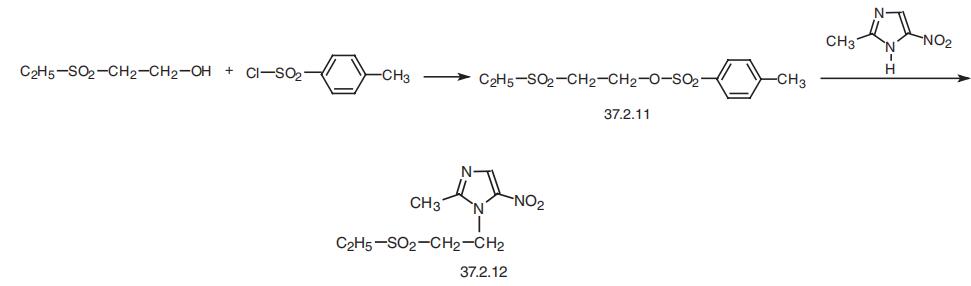
Synthesis
Tinidazole, 1-2-(ethylsulfonyl)ethyl-2-methyl-5-nitroimidazole (37.2.12), is
also made from 2-methyl-5-nitroimidazole (37.2.9), which upon being reacted with
2-ethoxysulfonyl-p-toluenesulfonate (37.2.11) transformed into the desired tinidazole.
The 2-ethoxysulfonyl-p-toluenesulfonate (37.2.11) necessary for this reaction is in turn
made by tosylation of 2-ethylsulfonyl ethanol using p-toluenesulfonyl chloride.
Veterinary Drugs and Treatments
Little information is presently available on the use of tinidazole in dogs, cats, or horses. It potentially could be useful for treating anaerobic infections, particularly associated with dental infections in small animals. Because of its antiprotozoal effects, it has been used as an alternative for treating giardiasis in small animals, and it could have efficacy against amebiasis, trichomoniasis or balantidiasis in veterinary species, but documentation of efficacy is not available. Tinidazole has a longer duration of action in dogs and cats than does metronidazole. In humans, oral tinidazole is FDA-approved for treating extraintestinal and intestinal amebiasis, (Entamoeba histolytica), giardiasis (Giardia duodenalis/lamblia), and trichomoniasis (T. vaginalis).
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: disulfiram-like reaction.
Metabolism
Hepatic, mainly via CYP3A4. Tinidazole, like metronidazole, is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation and conjugation. Tinidazole is the major drug-related constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite.
Metabolism
Tinidazole is excreted by the liver (up to 5
%) and kidneys
as unchanged drug and metabolites. An active hydroxy
metabolite has been identified.
Properties of Tinidazole
| Melting point: | 118-120?C |
| Boiling point: | 528.4±30.0 °C(Predicted) |
| Density | 1.4338 (rough estimate) |
| refractive index | 1.6320 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Practically insoluble in water, soluble in acetone and in methylene chloride, sparingly soluble in methanol. |
| form | neat |
| pka | 2.30±0.34(Predicted) |
| form | Solid |
| color | Off-White to Pale Yellow |
| λmax | 317nm(H2O)(lit.) |
| Merck | 14,9447 |
| BRN | 618182 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 19387-91-8(CAS DataBase Reference) |
Safety information for Tinidazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tinidazole
| InChIKey | HJLSLZFTEKNLFI-UHFFFAOYSA-N |
Tinidazole manufacturer
Remedy Labs
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![1-[2-(ethylthio)ethyl]-2-methyl-5-nitro-1H-imidazole](https://img.chemicalbook.in/CAS/GIF/28795-33-7.gif)
![[2-(ethylsulfonyl)ethyl]amine hydrochloride](https://img.chemicalbook.in/CAS/20180527/GIF/595596-97-7.gif)
You may like
-
 Tinidazole 99%View Details
Tinidazole 99%View Details -
 Tinidazole 19387-91-8 98%View Details
Tinidazole 19387-91-8 98%View Details
19387-91-8 -
 Tinidazole 19387-91-8 98%View Details
Tinidazole 19387-91-8 98%View Details
19387-91-8 -
 19387-91-6 TINIDAZOLE 99%View Details
19387-91-6 TINIDAZOLE 99%View Details
19387-91-6 -
 Tinidazole CAS 19387-91-8View Details
Tinidazole CAS 19387-91-8View Details
19387-91-8 -
 Tinidazole 97% CAS 19387-91-8View Details
Tinidazole 97% CAS 19387-91-8View Details
19387-91-8 -
 Tinidazole Powder API, 1KgView Details
Tinidazole Powder API, 1KgView Details
19387-91-8 -
 Tinidazole API Powder IP/BP/USP/EPView Details
Tinidazole API Powder IP/BP/USP/EPView Details
19387-91-8
