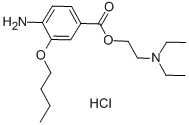
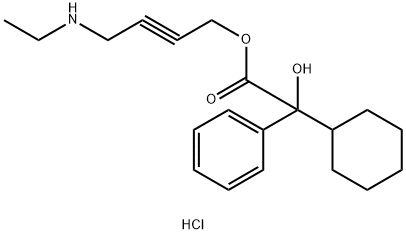
Benoxinate Hydrochloride
Synonym(s):2-(Diethylamino)ethyl 4-amino-3-butoxybenzoate hydrochloride;4-Amino-3-butoxybenzoic acid diethylaminoethyl ester;Benoxinate hydrochloride;Oxybuprocaine hydrochloride
- CAS NO.:5987-82-6
- Empirical Formula: C17H29ClN2O3
- Molecular Weight: 344.88
- MDL number: MFCD00012512
- EINECS: 227-808-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is Benoxinate Hydrochloride?
Chemical properties
White or almost white, crystalline powder or colourless crystals.
Originator
Dorsacaine HCl,Dorsey,US,1953
The Uses of Benoxinate Hydrochloride
Benoxinate Hydrochloride is a local anesthetic used in ophthalmology to numb the surface of the eye for a variety of procedures.
The Uses of Benoxinate Hydrochloride
antibacterial, coccidiostat
The Uses of Benoxinate Hydrochloride
anticonvulsant
Definition
ChEBI: The monohydrochloride salt of oxybuprocaine.
Manufacturing Process
25 grams of 3-oxy-4-nitrobenzoic acid are esterified (ethyl ester) and 26
grams of the ester are dissolved in 200 cc of absolute ether and treated with
7 grams of caustic potash in 20 cc of absolute methanol. The red potassium
phenolate with 7 grams of pure butyl bromide and 7 grams of absolute alcohol
are heated for 5 hours in the oven to 150°C. When cool, the alcohol is
evaporated in vacuo and the butoxy-nitrobenzoic acid ethyl ester is
precipitated with water. The substance is sucked off and saponified for 15
minutes with a solution of 2.5 grams of caustic potash in 30 cc of alcohol on a
water bath. The alcohol is evaporated in vacuo and the 3-butoxy-4-
nitrobenzoic acid is precipitated with hydrochloric acid. It forms needles which
melt at 174°C. 7.9 grams of dry acid are boiled for 45 minutes under a reflux
condenser with 25 cc of thionyl chloride. The excess of thionyl chloride is then
removed in vacuo, and the oil is distilled. The acid chloride has a yellow color
and solidifies.
7.3 grams of the acid chloride are treated with 6.6 grams of diethyl-aminoethanol
in 20 cc of absolute benzene. The mixture is then warmed for 1 hour
on a water bath. When cold, it is treated with a solution of soda and washed
with ether. After drying over potash, the ether and benzene are removed by
distillation and 3-butoxy-4-nitrobenzoic acid diethylamino-ethyl ester is
obtained, having a BP 215°C/2.5 mm.
5.0 grams of this product are hydrogenated in absolute alcohol solution with
fresh Raney nickel. When the absorption of hydrogen ceases (5 hours), the
solution is filtered and the alcohol evaporated in vacuo. The 3-butoxy-4-
aminobenzoic acid diethyl-amino-ethyl ester boils at 215°-218°C at 2 mm
pressure; it is an almost colorless oil.
By precipitation of a solution of the ester in absolute ether with hydrogen
chloride gas, the dihydrochloride is obtained; upon recrystallization from
alcohol/ether, it forms crystals which melt at 196°-197°C.
brand name
Dorsacaine (Novartis).
Therapeutic Function
Local anesthetic
Clinical Use
Benoxinate hydrochloride is a topical anesthetic. Benoxinate hydrochloride ophthalmic solution, 0.4%, was originally permitted in 1953 as Dorsacaine under NDA 08-729. The product was reviewed as part of the Drug Efficacy Study Implementation (DESI), found to be effective, approved and marketed by Dorsey until 1980. An abbreviated new drug application (ANDA) was also approved [Sola Barnes Hind (ANDA 84-149)] and marketed until 1991.
Side Effects
The most common ocular adverse events associated with Benoxinate Hydrochloride when used as an eye drop ingredient (Fluorescein and Benoxinate Ophthalmic Solution) were: stinging, burning and conjunctival redness. Serious adverse reactions include corneal toxicity and corneal damage due to insensitivity. Other possible side effects include eye congestion, burning, stinging, eye irritation, blurred vision and punctate keratitis.
Safety Profile
Poison by subcutaneous route. A topical anesthetic. When heated to decomposition it emits toxic fumes of NOx and HCl.
structure and hydrogen bonding
Oxybuprocaine hydrochloride acts as a local anesthetic and is used in eye drop formulations. Mod. II, the stable polymorphic form, contains two molecules with markedly different conformations (stretched and bent). The 13C CPMAS NMR spectrum of this sample12 showed crystallographic splittings arising from the fact that there are two molecules in the asymmetric unit. An INADEQUATE two-dimensional experiment was used to link signals for the same independent molecule. Of the four ethyl groups attached to NHt nitrogens, one gives rise to unusually low chemical shifts, very different from those of the other three groups. This was attributed to gamma-gauche conformational effects, and confirmed by shielding computations. The oxybuprocaine system is too large for computations performed on the crystallographic unit and the intermolecular shielding effects have to be neglected. Nevertheless, the computed shifts do match the order of the experimental ones. The assignment of 13C signals to specific carbons in the two crystallographically inequivalent molecules of oxybuprocaine polymorph showed the power of NMR crystallography.
Properties of Benoxinate Hydrochloride
| Melting point: | 157-160 C |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, freely soluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Merck | 13,1045 |
| InChI | InChI=1S/C17H28N2O3.ClH/c1-4-7-11-21-16-13-14(8-9-15(16)18)17(20)22-12-10-19(5-2)6-3;/h8-9,13H,4-7,10-12,18H2,1-3H3;1H |
| CAS DataBase Reference | 5987-82-6(CAS DataBase Reference) |
Safety information for Benoxinate Hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benoxinate Hydrochloride
| InChIKey | PRGUDWLMFLCODA-UHFFFAOYSA-N |
| SMILES | C1(C(=O)OCCN(CC)CC)=CC=C(N)C(OCCCC)=C1.Cl |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 5987-82-6 Oxybuprocaine Hydrochloride / Benoxinate Hydrochloride 98%View Details
5987-82-6 Oxybuprocaine Hydrochloride / Benoxinate Hydrochloride 98%View Details
5987-82-6 -
 5987-82-6 98%View Details
5987-82-6 98%View Details
5987-82-6 -
 Oxybuprocaine hydrochloride 95% CAS 5987-82-6View Details
Oxybuprocaine hydrochloride 95% CAS 5987-82-6View Details
5987-82-6 -
 Benoxinate hydrochloride CAS 5987-82-6View Details
Benoxinate hydrochloride CAS 5987-82-6View Details
5987-82-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
