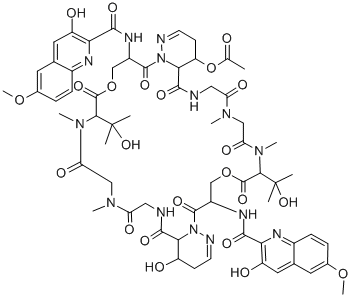
BBM-928 A
- CAS NO.:75580-37-9
- Empirical Formula: C64H78N14O24
- Molecular Weight: 1427.38
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-26 08:28:49
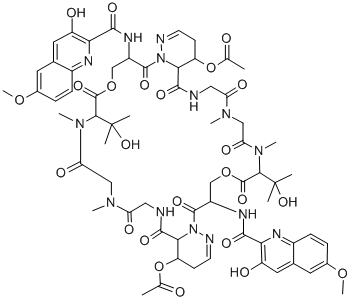
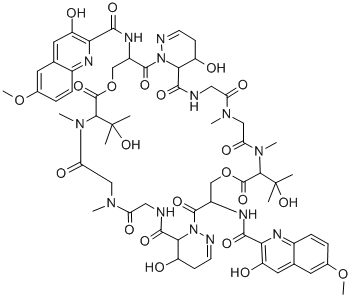
What is BBM-928 A?
The Uses of BBM-928 A
Luzopeptin A is a member of the echinomycin (quinomycin A) class of antitumor and antibiotic agents that act by forming a stable DNA complex by bis-intercalation. Luzopeptin A is alsoa potent inhibitor of HIV-1 reverse transcriptase.
The Uses of BBM-928 A
Luzopeptin A is a cyclic depsipeptide antibiotic first isolated from an actinomycete strain. It displays antitumor activity, as it is highly active in mice against a variety of experimental tumors. Luzopeptin A acts as a bifunctional DNA intercalator that strongly binds DNA and forms crosslinks between DNA molecules. It also inhibits reverse transcriptases from HIV-1 and HIV-2 (IC50s = 7 and 68 nM, respectively), as well as cellular DNA polymerases.[Cayman Chemical]
The Uses of BBM-928 A
Luzopeptin A is an inhibitor of HIV-1 and HIV-2 reverse transcriptase.
What are the applications of Application
Luzopeptin A is an inhibitor of HIV-1 and HIV-2 reverse transcriptase
Biological Activity
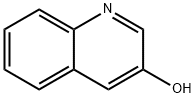
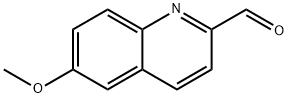
luzopeptin a is a cyclic depsipeptide antibiotic.a depsipeptide is a peptide in which one or more of its amide groups are replaced by the corresponding ester, or more generally, is a molecule that has both peptide and ester linkages in proximity in the same amino acid-containing small molecule or chain.
in vitro
previous study found that luzopeptin a treatment could produce additional dna bands which were the products of type ii biintercalation. the types of restriction fragments involved were identified. maximal type ii biintercalation occurred at a luzopeptin a/dna range of 0.14 to 0.18, at which more than 50% of the total dna molecules were involved. type ii products were gradually converted to type i products upon prolonged incubation at 37 degrees, maybe due to the tendency for intermolecular bonds to disrupt. echinomycin treatment failed to produce type ii products, probably because of a dna-binding affinity weaker than that of luzopeptin a [1].
in vivo
animal study showed that when administered as a suspension in 0.9% nacl solution, luzopeptin a demonstrated good activity against i.p. b16 melanoma and i.p. p388 leukemia and weak activity versus i.v. p388, i.p. l1210 leukemia, lewis lung, and madison 109 lung carcinomas. in terms of tumor cell kill, luzopeptin a induced net reductions in the body burdens of l1210 and p388 leukemias following single-drug injections but failed to yield net reductions following multiple-injection therapies [2].
References
[1] huang, c. h.,mirabelli, c.k.,mong, s., et al. intermolecular cross-linking of dna through bifunctional intercalation of an antitumor antibiotic, luzopeptin a (bbm-928a). cancer research 43, 2718-2724 (1983).
[2] rose wc, schurig je, huftalen jb, bradner wt. experimental antitumor activity and toxicity of a new chemotherapeutic agent, bbm 928a. cancer res. 1983 apr;43(4):1504-10.
Properties of BBM-928 A
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO;Soluble in dimethyl formamide |
| form | solid |
| pka | 6.75±0.40(Predicted) |
| color | tan |
Safety information for BBM-928 A
Computed Descriptors for BBM-928 A
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0