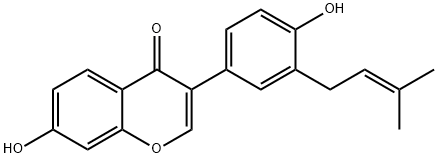
BAVACHIN
Synonym(s):(2S)-2,3-Dihydro-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one;4′,7-Dihydroxy 6-(3-methyl 2-butenyl)flavanone;Corylifolin
- CAS NO.:19879-32-4
- Empirical Formula: C20H20O4
- Molecular Weight: 324.37
- MDL number: MFCD11617359
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-10 18:54:13
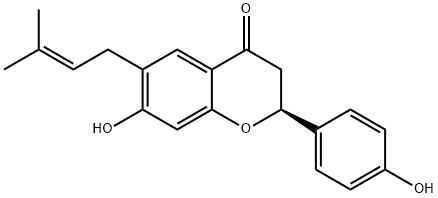
What is BAVACHIN?
Description
Bavachin is a flavonoid first isolated from seeds of P. corylifolia. It is a phytoestrogen that activates the estrogen receptors ERα and ERβ (EC50s = 320 and 680 nM, respectively). Through this action, bavachin stimulates osteoblast proliferation and differentiation and prevents bone loss following ovariectomy in rats. Bavachin less potently inhibits acyl-coenzyme A:cholesterol acyltransferase (IC50 = 86 μM).
The Uses of BAVACHIN
Bavachin has therapeutic potential for type 2 diabetes by activating insulin signaling pathways. Also, it has anabolic and potent anticatabolic biological effects on chondrocytes. Strong inhibitor of human UDP-glucuronosyltransferase (UGT1A1)
What are the applications of Application
Bavachin is a weak antioxidant that stimulates bone formation
References
[1] choi j h, rho m c, lee s w, et al. bavachin and isobavachalcone, acyl-coenzyme a: cholesterol acyltransferase inhibitors from psoralea corylifolia[j]. archives of pharmacal research, 2008, 31(11): 1419-1423.
[2] park j, kim d h, ahn h n, et al. activation of estrogen receptor by bavachin from psoralea corylifolia[j]. biomolecules & therapeutics, 2012, 20(2): 183-188.
[3] lee h, li h, noh m, et al. bavachin from psoralea corylifolia improves insulin-dependent glucose uptake through insulin signaling and ampk activation in 3t3-l1 adipocytes[j]. international journal of molecular sciences, 2016, 17(4): 527.
Properties of BAVACHIN
| Boiling point: | 558.3±50.0 °C(Predicted) |
| Density | 1.244 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≤20mg/ml in ethanol;30mg/ml in DMSO;50mg/ml in dimethyl formamide |
| form | crystalline solid |
| pka | 8.27±0.40(Predicted) |
| color | White |
| CAS DataBase Reference | 19879-32-4 |
Safety information for BAVACHIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for BAVACHIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Bavachin CAS 19879-32-4View Details
Bavachin CAS 19879-32-4View Details
19879-32-4 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1