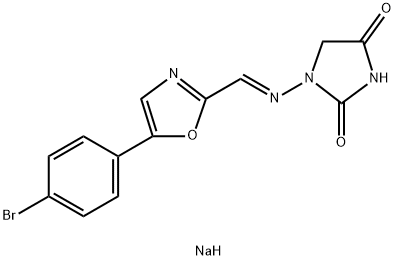
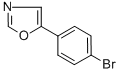
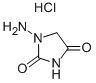
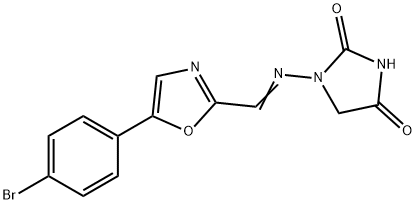
Azumolene
Synonym(s):1-[[[5-(4-Bromophenyl)-2-oxazolyl]methylene]amino]-2,4-imidazolidinedione;EU 4093
- CAS NO.:64748-79-4
- Empirical Formula: C13H9BrN4O3
- Molecular Weight: 349.14
- MDL number: MFCD00867710
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Azumolene?
Originator
Azumolene sodium,ZYF Pharm Chemical
The Uses of Azumolene
Relaxant (skeletal muscle).
Definition
ChEBI: Azumolene is a 1,3-oxazole which is substituted by a [(2,4-dioxoimidazolidin-1-yl)imino]methyl group at position 2 and a 4-bromophenyl group at position 5. It is a muscle relaxant used for the treatment/prevention of malignant hyperthermia. It has a role as a ryanodine receptor modulator and a muscle relaxant. It is a member of 1,3-oxazoles, a member of bromobenzenes and an imidazolidine-2,4-dione. It is a conjugate acid of an azumolene(1-).
Manufacturing Process
To a stirred solution of 2-bromo-4'-bromoacetophenone (100.0 g, 0.36 mole)
in chloroform (500 ml) was added hexamethylenetetramine (50.0 g, 0.36
mole). The mixture was stirred for 2.5 h and 143.0 g of the product was
collected by filtration (100%).
The product above was combined with a solution of methanol (300 ml) and
conc. HCl (410 ml), and the mixture was stirred for 52 h. The solid was
collected by filtration and was washed with isopropanol. The product was
recrystallized from methanol (Darco) to give 55.0 g (61%, in three crops),
melting point 284°-287°C.
To a stirred mixture of the above amine hydrochloride (55.0 g, 0.22 mole) and
[[(2,4-dioxo-1-imidazolidinyl)imino]methyl]formyl chloride (42.0 g, 0.22 mole)
was added a solution of 440 ml of dimethylformamide and 44 ml of pyridine.
The mixture was stirred for 20 h and poured into 2 L of water. The solid was
collected by filtration and washed with ethanol and ether to give 36.0 g (28%)
of N-[2-(4-bromophenyl)-2-oxoethyl]-[[(2,4-dioxo-1-imidazolidinyl)-
imino]methyl]formamide, melting point 267°-269°C (recrystallization from
2200 ml acetic acid).
The of N-[2-(4-bromophenyl)-2-oxoethyl]-[[(2,4-dioxo-1-imidazolidinyl)
imino]methyl]formamide (22.0 g, 0.061 mole) was combined with phosphorus
oxychloride (310 ml) and the mixture was stirred and refluxed for 7 h. The
solid was filtered off and stirred into an ice and water mixture (1 L). The 15.0
g (70%) of 1-[[[5-(4-bromophenyl)-2-oxazolyl]methylene]amino]-2,4-
imidazolidinedione was collected by filtration, melting point 290°-292°C
(recrystallization from 700 ml acetic acid).
Therapeutic Function
Muscle relaxant
Biochem/physiol Actions
Azumolene, a more water-soluble analog of dantrolene, is a potent inhibitor of sarcoplasmic reticulum (SR) ryanodine receptor (RyR) that inhibits skeletal muscle SR Ca2+ release. Azumolene is a direct acting skeletal muscle relaxant that induces effective blockade of skeletal muscle RyR1.
Properties of Azumolene
| storage temp. | Store at -20°C |
| solubility | DMSO: 13.89 mg/mL (39.78 mM) |
| form | Solid |
| color | White to light brown |
Safety information for Azumolene
Computed Descriptors for Azumolene
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
You may like
-
 Azumolene CAS 64748-79-4View Details
Azumolene CAS 64748-79-4View Details
64748-79-4 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2