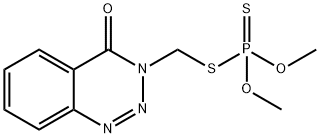
AZINPHOS-METHYL
Synonym(s):Guthion;Methyltriazotin
- CAS NO.:86-50-0
- Empirical Formula: C10H12N3O3PS2
- Molecular Weight: 317.32
- MDL number: MFCD00041814
- EINECS: 201-676-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
What is AZINPHOS-METHYL?
Description
Azinphos-methyl is a brown, waxy solid, orcolorless, crystalline material. Its technical form is a brownwaxy solid. Molecular weight= 317.34; Freezing/Meltingpoint=73°74℃; Vapor pressure= 2.0 3 10 2 7 mmHg.Hazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 0, Reactivity 0. Practicallyinsoluble in water.
Chemical properties
Azinphos methyl is an organophosphate pesticide available as a white or colorless crystalline solid substance. Technical azinphos methyl is a brown waxy solid that is sparingly soluble in water, but very soluble in most organic solvents. It is incompatible with strong oxidizing agents, and is hydrolyzed by water, acids, and alkalies. Azinphos methyl is used on many crops, especially apples, pears, cherries, peaches, almonds, and cotton for the control of a variety of crop pests and fruit crop pests. These include, codling moth, light brown apple moth, spring beetle, apple leaf hopper, bryobia mite, pear and cherry slug, woolly aphid, mites, codling moths, lygus-bugs, bollworms, armyworms, boll weevils, thrips, grasshoppers, stinkbugs, spittle bugs, plum curculio, pear psylla, scale, and the European brown snail. The use of azinphos methyl is not recommended either for public health or domestic purposes. Azinphos methyl is absorbed from the gastrointestinal tract, through the intact skin, and by inhalation of fi ne spray mist or dusts during spray operations. It is available as an emulsifi able liquid, a fl owable liquid, a ULV liquid, and wettable powder formulations.
Chemical properties
Pure azinphos-methyl is a colorless-to-white odorless crystalline solid. Azinphos-methyl is subject to hydrolysis and decomposes with gas evolution at elevated temperatures.Soluble in methanol, ethanol, propylene glycol, xylene, and other organic solvents; slightly soluble in water (33 mg/L).
The Uses of AZINPHOS-METHYL
Azinphos-methyl is used to control a wide range of both sucking and chewing insects and spider mites in a wide range of crops.
The Uses of AZINPHOS-METHYL
Insecticide for fruit. Use may be restricted.
The Uses of AZINPHOS-METHYL
Nonsystemic insecticide and acaricide for control of insects pests in blueberry, grape, maize, vegetable, cotton and citrus crops.
What are the applications of Application
Azinphos-methyl is an AChE inhibitor used as an insecticide
Definition
ChEBI: A member of the class of benzotriazines that is 1,2,3-benzotriazine substituted by an oxo group at position 4 and a [(dimethoxyphosphorothioyl)sulfanyl]methyl group at position 3.
General Description
Azinphos methyl is a colorless brown, waxy or white crystalline solid dissolved in a liquid carrier. AZINPHOS-METHYL is used as a pesticide. AZINPHOS-METHYL is added to water to create a water emulsifiable liquid. AZINPHOS-METHYL is toxic by inhalation, skin absorption, and/or ingestion. AZINPHOS-METHYL is heavier than and insoluble in water.
Technical-grade guthion is a cream to yellowbrown granular solid. Guthion is poorly soluble in water. Pure guthion is a colourless to white odourless crystalline solid. Technical-grade guthion is a cream to yellow-brown granular solid.
Air & Water Reactions
Insoluble in water. What little amount is solubilized will readily hydrolyze.
Reactivity Profile
The BPS Pesticide incident in Helena resulted in an explosion and death of three firemen. The burning of a 1,000 pound sack of Azinphos Methyl or the flashing of Maneb which was present on the facility may have caused the explosion. Azinphos Ethyl may behave similarly. At elevated temperatures, AZINPHOS-METHYL will decompose generating toxic gases.
Hazard
A poison, cholinesterase inhibitor. Absorbed by skin. Questionable carcinogen.
Health Hazard
Acute: extremely toxic. Probable oral lethal dose in humans is 5-50 mg/kg, or between 7 drops and 1 teaspoon for a 70 kg (150 lb.) person. A potent cholinesterase inhibitor which can cause death.
Health Hazard
Azinphos methyl is extremely toxic, the probable lethal oral dose for humans being from 5
to 50 mg/kg body weight (just seven drops). Different solvents used in the preparation and
commercial formulations of azinphos methyl further change the toxicological properties.
The symptoms of poisoning include, but are not limited to, excessive sweating, headache,
weakness, giddiness, nausea, hypersalivation, vomiting, stomach pains, blurred vision,
slurred speech, and muscle twitching, and with advanced time and poisoning, chest tightness,
vomiting, cramps, convulsion, coma, loss of refl exes and loss of sphincter control,
respiratory failure, unconsciousness, or death.
Reports have indicated that azinphos methyl causes no delayed neurotoxicity. Azinphos
methyl has been classifi ed as a tumorigen. After accidental or intentional exposure to
azinphos methyl, occupational workers exhibit over stimulation of the nervous system,
rapid twitching and paralysis of muscles, and death.
Health Hazard
Cholinesterase inhibitor; a severely acutetoxicant; delayed effects may be observedafter several hours; toxic symptoms simi-lar to other organophosphorus compounds;exposure may cause headache, dizziness,blurred vision, and muscle spasms. Othertoxic symptoms include vomiting, abdomi-nal pain, diarrhea, seizures, and shortness ofbreath; ingestion of 5–10 g could be fatalto adult humans; oral LD50 value (mice):15 mg/kg, rats 7 mg/kg; LC50 inhalation(rat): 69 mg/m3/1h.
Fire Hazard
Some of the formulations may burn, but none of them ignite easily. Container may explode in the heat of the fire. Rapidly hydrolyzed by cold alkali or cold acid. Unstable at temperatures above 390F.
Agricultural Uses
Insecticide, Acaricide: Not approved for use in EU countries. Azinphosmethyl liquids with a concentration greater than 13.5% are classified as a U.S. Restricted Use Pesticides (RUP) Insecticide used to control certain chewing and sucking insects in forestry settings, on fruits and fruit trees, vines, nuts, vegetables, cereals, maize, cotton, ornamentals, soy beans, tobacco, rice, coffee, sugar cane, and other crops. Also used as an intermediate in the manufacture, formulation and application of insecticides and acaricides. There are 31 global suppliers.
Trade name
ACIFON®; AZINPHOS-METHYL GUTHION®; BAY 9027®; BAYER 17147®; CARFENE®; COTNION-METHYL®; CRYSTHION 2 L®; CRYSTHYON®; DBD®; GOTHNION®; GUSATHION®; GUSATHION M®; GUTHION®; R 1582®
Safety Profile
Poison by inhalation, ingestion, skin contact, intravenous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. See also PARATHION and ESTERS. When heated to decomposition it emits very toxic fumes of POx, SOx, and NOx.
Potential Exposure
AgriculturalChemical; Tumorigen, Mutagen; Reproductive Effector.Personnel engaged in the manufacture, formulation, andapplication of this organophosphorus insecticide andacaricide.
First aid
: If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Speed in removing materialfrom skin is of extreme importance. Shampoo hairpromptly if contaminated. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is recommendedfor 24°48 h after breathing overexposure, as pulmonaryedema may be delayed. As first aid for pulmonary edema,a doctor or authorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
In a 2 year study dogs received azinphos-methyl technical (purity not stated) in the diet at 0, 5, 20, or 50 ppm . After 36 weeks, the 20 ppm and 50 ppm dose groups were given 50 and 100 ppm, respectively, upon lack of toxic symptoms. After 57 weeks, the 100 ppm dose group was elevated to 150 ppmand after 84weeks to 300 ppm. No evidence of carcinogenicity was observed.When azinphos-methyl was fed to mice at 0, 5, 20, or 40 ppm (about 0.79, 3.49, 11.33 (males); 0.98, 4.12, 14.30 (females)) for 2 years, no compound-related toxicity or evidence of cancer occurred .
Environmental Fate
Biological. Mixed cultures of microorganisms obtained from soil, raw sewage, acti vated sludge, and settled sludge were all able to degrade azinphosmethyl. When this
dithioate pesticide was incubated in a stirred flask containing a mixed culture for 4 days,
the concentration decreased from 99 to 40 mg/L (Barik et al., 1984).
Soil. The principal degradation products in soil and by selected soil microorganisms
are benzazimide, thiomethylbenzazimide, bis(benzazimidylmethyl)disulfide and anthra nilic acid. Benzazimide is further transformed only by Pseudomonas sp. DSM 50
When radiolabeled azinphos-methyl was incubated in soil, 50 and 93% of the applied
amount degraded to carbon dioxide after 44 and 197 days, respectively (Engelhardt et al.,
1984). The presence of benzamide, salicylic acid and 14CO2 from [carbonyl-14C]- an
In a dry soil (2–3% moisture content), the half-lives were 484, 88 and 32 days at 6,
25 and 40°C, respectively. In a soil containing 50% moisture, the half-lives were much
shorter, i.e., 64, 13 and 5 days at 6, 25 and 40°C, respectively (Yaron et al., 1974).
Azinphos-methyl will not leach to any great extent in soil (Helling, 1971). Staiff et
al. (1975) studied the persistence of azinphos-methyl in a test plot over an 8-year period.
At the end of the eighth year, virtually no azinphos-methyl was detected 30 c
Metabolic pathway
In the apple tree, 14C-azinphos methyl is metabolized to a small degree and, by the cell culture, 71% of the applied activity consists of unchanged azinphos methyl and its oxon is identified as a metabolite in a small amount. Two major metabolites are identified in the aqueous phase of the peel extract and in the cell extracts of the cell cultures. The major one is a conjugate of mercaptomethylbenzazimide with 2-(1- glucopyranosyl)propionic acid, and the minor one is monodesmethylazinphos methyl. In rats, following the administration of a single dose of azinphos methyl, the major radioactivity is eliminated in the expired air within 48 h. The metabolites in the urine result from the cleavage of P-S-C and P-O-CH3 bonds yielding O,O-dimethyl phosphorothioic acid together with mercaptomethylbenzamide and mono-O- demethylated azinphos methyl, respectively.
Storage
Azinphos methyl only should be handled by trained occupational workers/personnel wearing proper protective clothing. Azinphos methyl should always be stored and transported in clearly labeled impermeable containers under lock and key. Its storage area should be secure from access by unauthorized persons and children. Occupational workers must be properly informed that no food or drink should be stored in the storage areas.
Shipping
This chemical is an organophosphorus pesticide,solid, toxic. The required label is “POISONOUS/TOXICMATERIALS.” It falls in Hazard Class 6.1 and PackingGroup II.
Degradation
Azinphos-methyl is rapidly hydrolysed in alkaline and acidic media. It
rapidly photodegrades on soil surfaces and in water (PM).
The hydrolysis of [14C-carbonyl]azinphos-methywl as studied in buffered
aqueous solution, pH 6-11 and the decomposition products were
analysed by 2D TLC co-chromatography and GLC with authenticated
standards after seven days incubation. The compound was relatively stable
between pH 6 and 9 but at pH 10-11 it was largely decomposed. The
decomposition products were identified as 3-methylbenzazimide (2),
bis-3-methylbenzazimide disulfide (3), benzazimide (4), azinphos-methyl
oxon (5) and anthranilic acid (6). The proportion of the oxon was appreciable
at pH 9-10 but at pH 11 it was a minor component of the mixture.
When [14C-carbonyl]azinphos-methyl was subjected to elevated temperatures
on a glass surface in the dark for seven days it was found
to thermally degrade at appreciable rates at 50°C and above. The five
degradation products, which had also been found in the hydrolysis
experiments, were identified as products of thermal decomposition
with the addition that the intermediate 3-(thiomethyl)benzazimide (7)
was also characterised as well as the disulfide (3). Degradation was more
rapid in heated water than on glass. When [14C-carbonyl]azinphos-methyl
was subjected to irradiation as a water suspension or as a thin film by
UV light (254 nm), sunlight and red and yellow filtered light (intensities
unrecorded) for up to 12 hours the compound was rapidly degraded by
the UV light with 95% being decomposed after 60 min. Red or yellow light caused no photolysis and sunlight irradiation of a thin film on
glass caused some photodecomposition. Photolysis products identified
in the UV-irradiated samples were 3-methylbenzazimide (2), bis-3-
methylbenzazimide disulfide (3), benzazimide (4) and anthranilic acid (6)
in addition to four water-soluble products which were not identified.
Only benzazimide (4) and bis-3-methylbenzazimide disulfide (3) were
identified in the sunlight-irradiated sample (Liang and Lichtenstein, 1972).Routes for the hydrolysis, thermal decomposition and photolysis of azinphos-methyl are shown in Scheme 1.
Incompatibilities
Contact with oxidizers may cause therelease of phosphorous oxides. Contact with strong reducingagents, such as hydrides, may cause the formation of flammable and toxic phosphine gas.
Precautions
Heptachlor decomposes on heating above 160°C, producing toxic fumes including hydro- gen chloride. It reacts with strong oxidants and attacks metal. During use and handling of liquid and powder formulations of heptachlor, occupational workers should wear protec- tive neoprene or PVC gloves, cotton overalls, rubber boots, and a face shield or dust mask. Heptachlor should be kept stored in locked buildings.
Properties of AZINPHOS-METHYL
| Melting point: | 73℃ |
| Boiling point: | 421.3±55.0 °C(Predicted) |
| Density | 1.44 g/cm3 (20℃) |
| vapor pressure | 5.0 x 10-7 Pa (20 °C) |
| refractive index | 1.6115 (589.3 nm 76℃) |
| storage temp. | 0-6°C |
| form | solid |
| Water Solubility | 28 mg l-1(20 °C) |
| pka | -2.60±0.20(Predicted) |
| color | Crystals or brown, waxy solid |
| Merck | 13,915 |
| BRN | 280476 |
| Exposure limits | NIOSH REL: 0.2 mg/m3; OSHA PEL: TWA 0.2 mg/m3; ACGIH TLV:
TWA 0.2 mg/m3. |
| CAS DataBase Reference | 86-50-0(CAS DataBase Reference) |
| EPA Substance Registry System | Azinphos-methyl (86-50-0) |
Safety information for AZINPHOS-METHYL
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H311:Acute toxicity,dermal H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for AZINPHOS-METHYL
| InChIKey | CJJOSEISRRTUQB-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
