Azastene
- CAS NO.:13074-00-5
- Empirical Formula: C23H33NO2
- Molecular Weight: 355.519
- MDL number: MFCD00199200
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
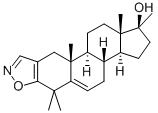
What is Azastene?
Originator
Azastene,ZYF Pharm Chemical
Manufacturing Process
A solution of 4,4,17α-trimethyl-androsta-2,5-dien-17β-ol-3-one in benzene
was added to sodium methoxide (from sodium and of absolute methanol,
concentrating the solution and drying the residue for 1 h at 150°-160°C and
15 mm). Ethyl formate was then added with stirring in a nitrogen atmosphere.
The reaction mixture was stirred for 4 h at room temperature, allowed to
stand for about 15 h, stirred for 2 h and then poured into water. The reaction
mixture was extracted with benzene, the aqueous layer warmed until clear,
filtered and cooled below room temperature. Concentrated hydrochloric acid
and ice were added to the filtrate until the mixture was acid to Congo red,
and the product was extracted with chloroform. The chloroform extracts were
washed with water, dried over anhydrous sodium sulfate, filtered and
concentrated vacuum, whereupon there separated 2-hydroxymethylene-
4,4,17α-trimethyl-androsta-2,5-dien-17β-ol-3-one.
To a solution of sodium acetate in acetic acid was added hydroxylamine
hydrochloride, and methanol was added until solution resulted. This solution
was added to a solution of 2-hydroxymethylene-4,4,17α-trimethyl-androsta-
2,5-dien-17β-ol-3-one in absolute methanol, and the combined solution was
refluxed for 40 min on a steam bath. The reaction mixture was concentrated,
the residue extracted with ethyl acetate, and the ethyl acetate extracts were
washed with ethyl acetate, and the ethyl acetate extracts were washed with
5% hydrochloric acid, dried over anhydrous sodium sulfate and concentrated
whereupon there separated solid material.
The mother liquors were concentrated to dryness and the residue
recrystallized from ether to give solid product. The latter was
chromatographed on a column of silica gel in benzene solution, recrystallized
from ethyl acetate and dried at 75°C for 20 h to give 17β-hydroxy-4,4,17α-
trimethyl-androsta-2,5-dien[2,3-d]isoxazole.
The crude product was chromatographed on silica gel in benzene solution,
eluted with benzene containing 5% of ether and recrystallized from ethanol to
give 17β-hydroxy-4,4,17α-trimethyl-androsta-2,5-dien[2,3-d]isoxazole.
Therapeutic Function
Contraceptive
Properties of Azastene
| Melting point: | 178.5°C |
| Boiling point: | 488.52°C (rough estimate) |
| Density | 1.14 |
| refractive index | 1.5614 (estimate) |
| pka | 15.13±0.70(Predicted) |
Safety information for Azastene
Computed Descriptors for Azastene
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1