Azaloxan
- CAS NO.:72822-56-1
- Molecular Weight: 0
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
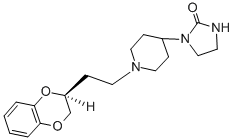
What is Azaloxan?
Originator
Idulian,Unicet
The Uses of Azaloxan
Antidepressant.
Manufacturing Process
The producing of starting materials 1 and 2:
1). The solution of 16.0 g of bromine in 10 ml of petroleum ether is slowly
added to the solution of 6.7 g of allyl cyanide in 30 ml of the same solvent,
while stirring and keeping the temperature at about -15°C. After removal of
the solvent the oily 3,4-dibromo-butyronitrile is obtained [J.A. C.S. 67, 400
(1945)].
227.0 g of 3,4-dibromobutyronitrile are added dropwise in 5 equal parts to the
stirred mixture of 85.0 g of catechol and 50. g of anhydrous potassium
carbonate in 100 ml of refluxing acetone each. Another 50.0 g of potassium
carbonate are added, followed by a slow addition of another part of nitrile.
After 3 more cycles, using 40.0 g of potassium carbonate, 1 part nitrile each
and sufficient acetone to allow stirring, the mixture is refluxed for 20 h. It is filtered, the filtrate evaporated, the residue distilled and the fraction boiling at
105°C/0.15 mm Hg collected, to yield the 1,4-benzodioxan-2-yl-acetonitrile
[Belgium Pat. No. 643,853-Aug. 14, 1964].
The mixture of 111.0 g of 1,4-benzodioxan-2-yl-acetonitrile, 63.5 ml of
sulfuric acid, 160 ml of acetic acid and 160 ml of water is refluxed for 48 h. It
is poured on ice, the resulting solid collected to yield the 1,4-benzodioxan-2-
yl-acetic acid, melting point 100°C, (recrystallized from benzene-petroleum
ether) [Belgium Pat. No. 613,211-July 30, 1962].
The solution of 5.8 g of 1,4-benzodioxan-2-yl-acetic acid in 100 ml of benzene
is added dropwise to 16.5 ml of a refluxing, 70% benzene solution of sodium
bis(2-methoxyethoxy)aluminum hydride under nitrogen. When addition is
complete, the mixture is refluxed for 4 h, cooled and poured slowly into 20 ml
of 25% sulfuric acid. After filtration and removal of the solvent, the residue is
taken up in methylene chloride, the solution washed several times with
saturated aqueous sodium bicarbonate, dried and evaporated, to yield the oily
2-(2-hydroxyethyl)-1,4-benzodioxan.
The mixture of 3.6 g of 2-(2-hydroxyethyl)-1,4-benzodioxan, 5.7 g of ptoluenesulfonyl
chloride and 20 ml of dry pyridine is stirred and cooled in an
ice bath for 2 h. Ice is then added to the mixture, the resulting solid is filtered
off to yield the 2-(2-tosyloxyethyl)-1,4-benzodioxan, melting point 82°-83°C
(recrystallized from ethyl acetate-petroleum ether).
2). To the solution of 1.6 g of 4-aminopyridine in 7 ml of dimethylformamide
2.0 g of 2-chloroethylisocyanate are added while stirring and keeping the
temperature below 40°C. After 2 h 28 ml of water are added and stirring is
continued for 2 h at room temperature. The precipitate formed is filtered off,
washed with water, dried to yield the 1-(4-pyridyl)-3-(2-chloroethyl)urea,
melting point 120°-122°C, (recrystallized from aqueous ethanol).
To the suspension of 2.66 g of 1-(4-pyridyl)-3-(2-chloroethyl)urea in 4 ml of
boiling methanol, 2.68 g of 30.8% methanolic sodium methanolate are added
while stirring and the mixture is refluxed for 1 h. It is filtered hot, washed
with hot methanol, the filtrate evaporated, to yield the 1-(4-pyridyl)-2-
imidazolidinone, melting point 204°-207°C, (recrystallized from 90% aqeuous
ethanol).
The solution of 5.0 g of 1-(4-pyridyl)-2-imidazolidinone in 45 ml of water is
hydrogenated over 0.8 g of 10% ruthenium on carbon at 120°C and 120 atm
until the hydrogen absorption ceases. It is filtered, the filtrate evaporated, the
residue taken up in chloroform, the solution dried, evaporated to yield the 1-
(4-piperidyl)-2-imidazolidinone, melting point 155°-157°C (recrystallized from
methylene chloride-petroleum ether).
The producing of (cis)-(S)-1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]-
2-imidazolidinone fumarate:
The mixture of 4.9 g of 2-(2-tosyloxyethyl)-1,4-benzodioxan, 2.54 g of 1-(4-
piperidyl)-2-imidazolidinone, 5.0 g of anhydrous sodium carbonate and 100 ml
of 4-methyl-2-pentanone is stirred and refluxed for 3 days. It is filtered,
evaporated, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]-2-
imidazolidinone, melting point 125°C, (recrystallized from isopropanol).
To the solution of 2.0 g of 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]-
2-imidazolidinone in the mini-mum amount of ethanol, the saturated solution
of 0.78 g of furmaric acid in boiling ethanol is added. The mixture is cooled to
0°C and the precipitate collected, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)-
ethyl]-4-piperidyl]-2-imidazolidinone fumarate, melting point 190°C.
The (cis)-(S)-form may be produced by chromatographic division of isomers of
1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]-2-imidazolidinone fumarate.
Therapeutic Function
Antidepressant
Safety information for Azaloxan
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4
![2-(2,3-DIHYDRO-BENZO[1,4]DIOXIN-2-YL)-ETHYLAMINE](https://img.chemicalbook.in/CAS/GIF/87086-36-0.gif)