Azacosterol
- CAS NO.:313-05-3
- Empirical Formula: C25H44N2O
- Molecular Weight: 388.63
- MDL number: MFCD00867156
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
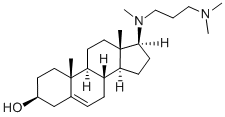
What is Azacosterol?
Originator
Ornitrol,Avitrol Corporation
The Uses of Azacosterol
Chemosterilant, avian.
Manufacturing Process
A solution of 15 parts of 3β-hydroxyandrost-5-en-17-one and 30 parts of 3-
dimethylaminopropylamine in 36.6 parts of formic acid is heated in an oil bath
at about 170-180°C for about 24 hours. The cooled mixture is diluted with
about 500 parts of water, and the resulting aqueous mixture is extracted with
chloroform, containing a small amount of methanol. The organic layer is
separated, washed with water, dried over anhydrous sodium sulfate, and
evaporated to dryness under reduced pressure. The viscous residue is
dissolved in a mixture of 80 parts of isopropyl alcohol and 420 parts of ether,
and this solution is treated with isopropanolic hydrogen chloride. The resulting
precipitate is collected by filtration and washed with acetone to afford 17β-[N-
(3-dimethylaminopropyl)formamido-1-androst-5-en-3β-ol hydrochloride. A
solution of this hydrochloride in aqueous methanol is made alkaline by the
addition of dilute aqueous sodium hydroxide, and the resulting colloidal
precipitate is extracted with chloroform. The chloroform extracts washed with
water, dried over anhydrous sodium sulfate and concentrated to dryness to
afford a residue, which is crystallized from acetone, resulting in 17β-[N-(3-
dimethylaminopropyl)formamido]androst-5-en-3β-ol, which displays a double
melting point at about 116-118°C and 143-148°C, [α]D= -67.5° (chloroform).
To a slurry of 4 parts of LiAlH4 in 150 parts of dioxane is added dropwise with
stirring, at the reflux temperature a solution of 10 parts of 17β-[N-(3-
dimethylaminopropyl)
formamido]androst-5-en-3β-ol in 150 parts of dioxane. This reaction mixture
is heated at reflux for about 18 hours longer, and then treated dropwise
successively, at the reflux temperature, with a solution of 4 parts of water in
25 parts of dioxane, 3 parts of 20% aqueous sodium hydroxide, and 14 parts
of water. The resulting mixture is clarified by filtration, and the residue on the
filter is washed with fresh dioxane. The filtrates are combined, evaporated to
dryness under reduced pressure, and the resulting residue is recrystallized
from acetone-methanol to produce 17β-[N-methyl-N-(3-
dimethylaminopropyl)amino]androst-5-en-3β-ol, M.P. about 146-148°C, [α]D=
-54.5° (chloroform). A solution of this amine in ether-isopropyl alcohol is
treated with isopropanolic hydrogen chloride to afford the corresponding
dihydrochloride, [α]D= -32° (methanol).
Therapeutic Function
Hypocholesteremic
Properties of Azacosterol
| Melting point: | 146-148° |
| Boiling point: | 490.6±40.0 °C(Predicted) |
| alpha | D -54.5° |
| Density | 1.04 |
| pka | 15.02±0.70(Predicted) |
| EPA Substance Registry System | Azacosterol (313-05-3) |
Safety information for Azacosterol
Computed Descriptors for Azacosterol
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4