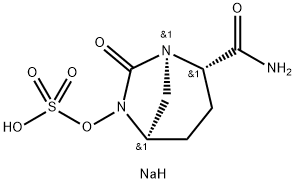
AvibactaM
- CAS NO.:1192500-31-4
- Empirical Formula: C7H11N3O6S
- Molecular Weight: 265.24
- MDL number: MFCD30478396
- EINECS: 685-962-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16

What is AvibactaM?
Toxicity
Avycaz is contraindicated in patients with known serious hypersensitivity to avibactam-containing products, ceftazidime or other members of the cephalosporin class.
The Uses of AvibactaM
Avibactam Butylammonium Salt is a novel β-lactamase inhibitor with a non-lactam structural scaffold. Avibactam irreversibly inhibits lactamase from Mycobacterium tuberculosis.
Background
Avibactam is a non-β-lactam β-lactamase inhibitor that is available in combination with ceftazidime (Avycaz). This combination was approved by the FDA on February 25, 2015 for the treatment of complicated intra-abdominal infections in combination with metronidazole, and the treatment of complicated urinary tract infections, including pyelonephritis caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant gram-negative bacterial pathogens. As there is limited clinical safety and efficacy data, Avycaz should be reserved for patients over 18 years old who have limited or not alternative treatment options.
Indications
AVYCAZ (ceftazidime-avibactam), in combination with metronidazole, is indicated for the treatment of complicated intra-abdominal infections caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Providencia stuartii, Enterobacter cloacae, Klebsiella oxytoca, and Pseudomonas aeruginosa in patients 18 years or older. AVYCAZ is also indicated for the treatment of complicated urinary tract infections including pyelonephritis caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter koseri, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freundii, Proteus spp., and Pseudomonas aeruginosa in patients 18 years or older.
Definition
ChEBI: A member of the class of azabicycloalkanes that is (2S,5R)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide in which the amino hydrogen at position 6 is replaced by a sulfooxy group. Used (in the form of its sodium salt) n combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis.
Enzyme inhibitor
This non-β-lactam inhibitor of β-lactamase (FW = 261.27 g/mol; CAS 1192500-31-4), also known as NLX104 and AVE1330A, shows a spectrum of action against Classes A and C β-lactamase as well as selected Class D β-lactamase, enzymes that often confer resistance to β-lactam antibiotics. By forming a high-affinity complex with its target enzymes, avibactam enhances the antibacterial activity of certain β-lactam drugs, such as ceftaroline. With over 1000 known β-lactamase, the action of avibactam is apt to depend on the invidual active-site interactions. Mode of Action: Acylation and deacylation rates have been measured for the clinically important enzymes CTX-M-15, KPC-2, E. cloacae AmpC, P. aeruginosa AmpC, OXA-10, and OXA-48. The efficiency of acylation (kon/Ki) varies across the enzyme spectrum, from 1.1 x 101 M–1 s –1 for OXA-10 to 1.0 x 105 M–1 s –1 for CTX-M-15. Inhibition of OXA-10 was shown to follow a reversible covalent mechanism (see below), and the acylated OXA-10 displayed the longest residence time for deacylation, with a half-life of greater than 5 days. The inhibited enzyme forms are stable to hydrolysis for all enzymes with the exception of KPC-2, which displays slow hydrolytic route that involved fragmentation of the acyl-avibactam complex. In the case of TEM-1 lactamase, avibactam slowly covalently acylates its target, and the acylated enzyme subsequently undergoes slow deacylation (koff = 0.045 min?1 ) regenerating avibactam intact. Use as a Combined Drug: Combination of avibactim with extended-spectrum cephalosporins or aztreonam shows promise in inhibiting Klebsiella pneumoniae (KP) isolates harboring carbapenemases, or KPCs. Given abundant experience with ceftazidime and the significant improvement avibactam provides against contemporary β-lactamase-producing Gram-negative pathogens, this combination will likely play a role in the treatment of complicated urinary tract infections (as monotherapy) and complicated intra-abdominal infections (in combination with metronidazole) caused (or suspected to be caused) by otherwise resistant pathogens, such as extended spectrum β-lactamase-, AmpC-, or Klebsiella pneumoniae carbapenemase producing Enterobacteriaceae and multidrug-resistant P. aeruginosa.
Metabolism
No metabolism of avibactam was observed in human liver preparations. Unchanged avibactam is the major drug-related component in human plasma and urine. 80-90% of ceftazidime is eliminated as unchanged .
Properties of AvibactaM
| Density | 1.85 |
| storage temp. | Store at -20°C |
| solubility | DMSO |
| form | Powder |
| pka | -4.59±0.18(Predicted) |
| color | White to off-white |
Safety information for AvibactaM
Computed Descriptors for AvibactaM
AvibactaM manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1