Avibactam sodium
- CAS NO.:1192491-61-4
- Empirical Formula: C7H12N3NaO6S
- Molecular Weight: 289.24
- MDL number: MFCD28900719
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
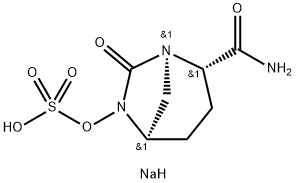
What is Avibactam sodium?
Description
Avibactam is a β-lactamase inhibitor (IC50s = 8, 80, and 38 nM for TEM-1, P99, and KPC-2 β-lactamases, respectively). It restores the antimicrobial activity of ceftazidime , ceftriaxone , imipenem , and piperacillin against antibiotic-resistant Enterobacteriaceae in vitro (MIC90 reduction 4-1,024-fold across 190 E. coli and K. pneumoniae clinical isolates). Formulations containing avibactam have been used to treat carbapenem-resistant Enterobacteriaceae infections.
Description
Avibactam sodium and its free acid, avibactam, are diazabicyclooctane (DBO)–based, non-β-lactam β-lactamase inhibitors (BLIs) that are used to treat multi-drug–resistant Gram-negative bacterial pathogens. The compounds were jointly developed by Actavis Generics (now part of Teva Pharmaceutical Industries) and AstraZeneca.??
In 2015, avibactam sodium, in combination with ceftazidime, a cephalosporin antibiotic, was approved by the US Food and Drug Administration. The dual-drug’s target was complex urinary tract and intra-abdominal infections caused by Gram-negative, often hospital-acquired, bacteria.
Last year, in the continuing quest to combat multi-drug–resistant bacteria, Eric M. Gordon, Matthew A. J. Duncton, and Mark A. Gallop at Arixa Pharmaceuticals (Palo Alto, CA) prepared analogues of avibactam that are more orally available than the free acid or its salt. (Currently, avibactam can be administered only intravenously; the only orally available BLI on the market is the less useful clavulanic acid.)
The researchers prepared prodrugs of avibactam in which various moieties form esters of the sulfate group. (Historically, charged sulfate groups on drug molecules were seen as strong obstacles to oral bioavailability.) The derivatives with the highest oral availabilities to laboratory animals had a quaternary carbon atom in the β position of the added sulfate ester and a carboxylate ester as one of the substituents on the quaternary carbon (i.e., –OSO3–CH2–CR1R2–CO2R3).
The authors believe that their avibactam analogues are orally available because of steric hindrance to hydrolysis provided by the sulfate ester and the gem-disubstituent/trimethyl lock effect that brings the carboxylate nucleophile closer to the eventual displacement site. In a simulation of in vivo conditions, they demonstrated that the prodrug can be cleaved under mild conditions to release the unesterified avibactam.
History
Avibactam sodium and its free acid, avibactam, are diazabicyclooctane (DBO)–based, non-β-lactam β-lactamase inhibitors (BLIs) that are used to treat multi-drug–resistant Gram-negative bacterial pathogens. The compounds were jointly developed by Actavis Generics (now part of Teva Pharmaceutical Industries) and AstraZeneca.
In 2015, avibactam sodium, in combination with ceftazidime, a cephalosporin antibiotic, was approved by the US Food and Drug Administration. The dual-drug’s target was complex urinary tract and intra-abdominal infections caused by Gram-negative, often hospital-acquired, bacteria.
In 2018, in the continuing quest to combat multi-drug–resistant bacteria, Eric M. Gordon, Matthew A. J. Duncton, and Mark A. Gallop at Arixa Pharmaceuticals (Palo Alto, CA) prepared analogues of avibactam that are more orally available than the free acid or its salt.
Characteristics
Avibactam sodium is a novel beta-lactamase inhibitor. In its structure, Na+ readily forms coordination bonds with water molecules. In addition, there are several O atoms as hydrogen bond acceptors and -NH2 as hydrogen bond donors. As a result, avibactam sodium crystallises easily into its hydrated form[1]. The structure is shown below:
The Uses of Avibactam sodium
Avibactam (NXL104) is a novel β-lactamase inhibitor with a non-lactam structural scaffold. NXL104 irreversibly inhibits lactamase from Mycobacterium tuberculosis.
Definition
ChEBI: Avibactam sodium is an organic sodium salt that is the monosodium salt of avibactam. Used in combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis. It has a role as an EC 3.5.2.6 (beta-lactamase) inhibitor, an antibacterial drug and an antimicrobial agent. It contains an avibactam(1-).
Biological Activity
Avibactam[1192491-61-4] is a β-lactamase inhibitor (IC50s = 8, 80, and 38 nM for TEM-1, P99, and KPC-2 β-lactamases, respectively). It restores the antimicrobial activity of ceftazidime , ceftriaxone , imipenem , and piperacillin against antibiotic-resistant Enterobacteriaceae in vitro (MIC90 reduction 4-1,024-fold across 190 E. coli and K. pneumoniae clinical isolates). Formulations containing avibactam have been used to treat carbapenem-resistant Enterobacteriaceae infections.
References
[1] STACHYRATHéRèSE. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor.[J]. Antimicrobial Agents and Chemotherapy, 2010: 5132-5138. DOI:10.1128/AAC.00568-10.
[2] BONNEFOYALAIN. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor.[J]. Journal of Antimicrobial Chemotherapy, 2004, 54 2: 410-417. DOI:10.1093/jac/dkh358.
[3] LAGACé-WIENSP R S. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases.[J]. Antimicrobial Agents and Chemotherapy, 2011, 55 5: 2434-2437. DOI:10.1128/AAC.01722-10.
[4] KINGMADELINE. Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections.[J]. Antimicrobial Agents and Chemotherapy, 2017, 61 7. DOI:10.1128/AAC.00449-17.
[5] ZHIYONG DING. Understanding the Role of Water in Different Solid Forms of Avibactam Sodium and Its Affecting Mechanism[J]. Crystal Growth & Design, 2020, 20 2: 1150-1161. DOI:10.1021/acs.cgd.9b01459.
References
[1] ZHIYONG DING. Understanding the Role of Water in Different Solid Forms of Avibactam Sodium and Its Affecting Mechanism[J]. Crystal Growth & Design, 2020. DOI:10.1021/acs.cgd.9b01459.
Properties of Avibactam sodium
| Melting point: | >208°C (dec.) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated), Water (Slightly, Sonica |
| form | Solid |
| appearance | white crystalline powder |
| color | White to Off-White |
| InChI | InChI=1/C7H11N3O6S.Na.H/c8-6(11)5-2-1-4-3-9(5)7(12)10(4)16-17(13,14)15;;/h4-5H,1-3H2,(H2,8,11)(H,13,14,15);;/t4-,5+;;/s3 |
Safety information for Avibactam sodium
Computed Descriptors for Avibactam sodium
| InChIKey | JKMUNBAKQJMVKG-NLZGLQCHNA-N |
| SMILES | C([C@@H]1CC[C@H]2N(OS(O)(=O)=O)C(=O)[N@@]1C2)(=O)N.[NaH] |&1:1,4,13,r| |
Avibactam sodium manufacturer
Sumar biotech LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![N-[[[2-(Aminooxy)ethyl]amino]sulfonyl]carbamic acid 1,1-dimethylethyl ester](https://img.chemicalbook.in/CAS/20180629/GIF/1452466-39-5.gif)
![ethyl (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxylate](https://img.chemicalbook.in/CAS/20180629/GIF/1383814-58-1.gif)
![tert-butyl ((2S)-1-(((2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamido)oxy)propan-2-yl)carbamate](https://img.chemicalbook.in/CAS/20180629/GIF/1452466-25-9.gif)
![4-[2-(Aminooxy)ethoxy]-1-piperidinecarboxylic acid 1,1-dimethylethyl ester](https://img.chemicalbook.in/CAS/20180702/GIF/1452466-34-0.gif)
![(2S,5R)-N'-acetyl-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carbohydrazide](https://img.chemicalbook.in/CAS/20180702/GIF/1452467-32-1.gif)
You may like
-
 1192491-61-4 Avibactam Sodium 98%View Details
1192491-61-4 Avibactam Sodium 98%View Details
1192491-61-4 -
 Avibactum Sodium 1192491-61-4 >98%View Details
Avibactum Sodium 1192491-61-4 >98%View Details
1192491-61-4 -
 1192491-61-4 Avibactam sodium 98%View Details
1192491-61-4 Avibactam sodium 98%View Details
1192491-61-4 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 1122421-c1-4 Avibactum Sodium >98%View Details
1122421-c1-4 Avibactum Sodium >98%View Details
1122421-c1-4 -
 Avibactam sodium 97% CAS 1192491-61-4View Details
Avibactam sodium 97% CAS 1192491-61-4View Details
1192491-61-4 -
 Avibactam Sodium CAS 1192491-61-4View Details
Avibactam Sodium CAS 1192491-61-4View Details
1192491-61-4 -
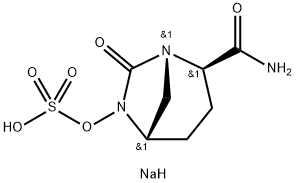
![Sodium(1r,2s,5r)2-Carbamoyl-7-Oxo-1,6-Diazabicyclo[3,2,1]Octan-6-Yl Sulfate](https://img.chemicalbook.in//ProductImageIndia/2025-09-22/20250918001660627552731400.jpg) Sodium(1r,2s,5r)2-Carbamoyl-7-Oxo-1,6-Diazabicyclo[3,2,1]Octan-6-Yl SulfateView Details
Sodium(1r,2s,5r)2-Carbamoyl-7-Oxo-1,6-Diazabicyclo[3,2,1]Octan-6-Yl SulfateView Details
1192491-61-4
