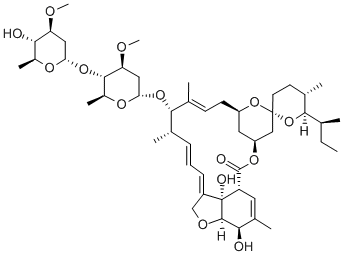
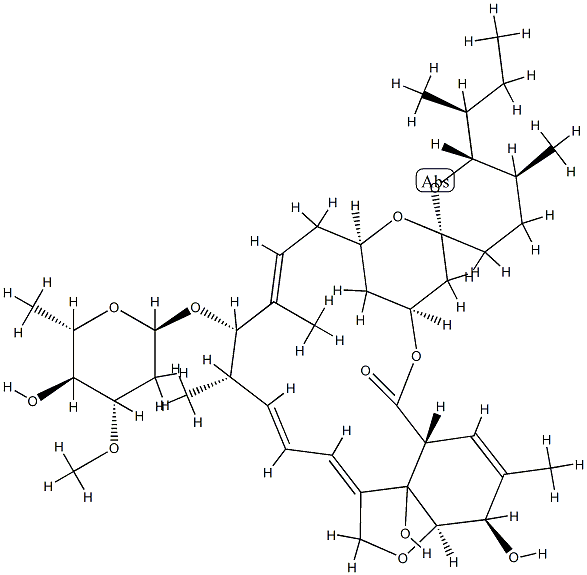
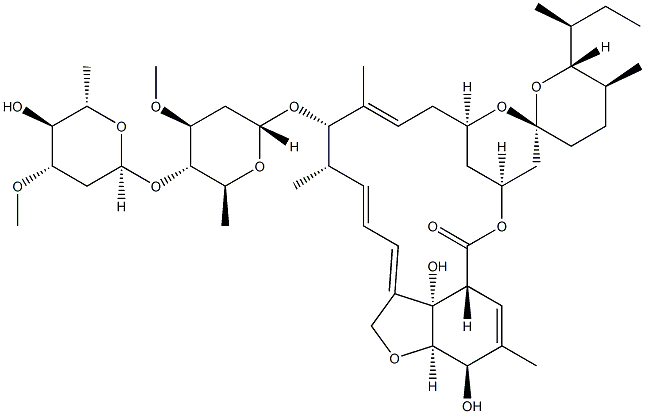
Avermectin B1a
- CAS NO.:65195-55-3
- Empirical Formula: C48H72O14
- Molecular Weight: 873.09
- MDL number: MFCD01939385
- EINECS: 265-610-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
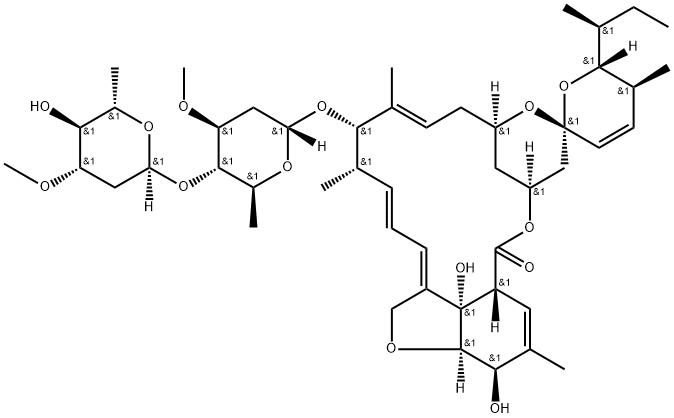
What is Avermectin B1a?
Description
Avermectin B1a is a macrocyclic lactone disaccharide anthelmintic agent that binds to high and low affinity sites on the mammalian GABAA receptor. Binding to the high affinity site activates the receptor to increase chloride influx, while binding to the low affinity site blocks the channel. Avermectin B1a inhibits binding of the glycine receptor antagonist strychnine to membranes and the solubilized receptor from rat spinal cord (Kis = 1.3 and 3.6 μM, respectively). Formulations containing avermectin B1a (>80%) and avermectin B1b (~20%; ) are used in insecticides and veterinary anthelmintic formulas as abamectin .
Originator
Abamectin,Yellow River Enterprise Co. (a.k.a Yelori)
The Uses of Avermectin B1a
Avermectin B1a is the major component (>80%) of a commercially available anthelmintic used to control parasitic nematodes in livestock. Avermectin B1a contains a secbutyl residue in the 25-position. In vitro and in vivo studies have shown that this analogue is the more potent analogue in the commercial product.
What are the applications of Application
Avermectin B1a is a macrocyclic lactone with insecticide and acaricide properties
Definition
ChEBI: Avermectin B1a is an avermectin.
Manufacturing Process
1. The contents of a lyophilized tube of Streptomyces avermitilis MA-4680 is
transferred aseptically to a 250 ml Erlenmeyer flask containing 305 ml of
Medium 1: Dextrose 20 g, Peptone 5 g, Meat Extract 5 g, Primary Yeast 3 g,
NaCl 5 g, CaCO3 (after pH adjustment) 3 g, Distilled water 1000 ml, pH 7.0.
The inoculated flask is incubated for 3 days at 28°C on a rotary shaking
machine at a speed of 220 RPM in a 2 inch radius circular orbit. At the end of
this time, a 250 ml Erlenmeyer flask containing 50 ml of Medium 2 [Tomato
Paste 20 g, Modified Starch (CPC) 20 g, Primary Yeast 10 g, CoCl2·6H2O 0.005
g, Distilled water 1000 ml, pH 7.2-7.4] is inoculated with a 2 ml sample from
the first flask. This flask is incubated for 3 days at 28°C on a rotary shaking
machine at a speed of 220 RPM in a 2 inch diameter circular orbit. 50 Ml of
the resulting fermentation broth containing C-076 is effective against an
N.dubius infection in mice.
2. A lyophilized tube of Streptomyces avermitilis MA-4680 is opened
aseptically and the contents suspended in 50 ml of Medium 1 in a 250 ml
Erlenmeyer flask. This flask is shaken for 3 days at 28°C on a rotary shaking
machine 220 RPM with a 2 inch diameter circular orbit. A 0.2 ml portion of
this seed medium is used to inoculate a Slant of Medium 3: Dextrose 10.0 g ,
Bacto Asparagine 0.5 g, K2HPO4 40.5 g, Bacto Agar 15.0 g , Distilled water
1000 ml, pH 7.0. The inoculated slant medium is incubated at 28°C for 10
days and stored at 4°C until used to inoculate 4 more slants of Medium 3.
These slants are incubated in the dark for 8 days. One of these slants is used
to inoculate 3 baffled 250 ml Erlenmeyer flasks containing 50 ml of No. 4
Seed Medium: Soluble Starch 10.0 g, Ardamine 5.0 g, NZ Amine E 5.0 g, Beef
Extract 3.0 g, MgSO4·7H2O 0.5 g, Cerelose 1.0 g, Na2HPO4 0.190 g, KH2PO4
182 g, CaCO3 0.5 g, Distilled water 1000 ml, pH 7.0-7.2. The seed flasks are
shaken for 2 days at 27-28°C on a rotary shaking machine at 220 RPM with a
2 inch diameter circular orbit. The contents of these flasks are pooled and
used to inoculate (5% inoculum) baffled 250 ml Erlenmeyer flasks containing
40 ml of various production media. Flasks containing media 2, 5 and 6 are
incubated for 4 days at 28°C on a rotary shaking machine at 220 RPM with a
2 inch diameter circular orbit. The resulting broth containing C-076 is then
harvested and tested for anthelmintic activity. In all cases 6.2 ml of whole
broth and the solids obtained from centrifuging 25 ml of whole broth are fully
active against N.dubius helminth infections in mice.
3. The one of the four slants of Medium 3 prepared as in Example 2 is used to
inoculate a baffled 250 ml Erlenmeyer flask containing 50 ml of Seed Medium
No. 4. The seed flask is shaken for 1 day at 27- 28°C on a rotary shaking
machine at 220 RPM with a 2 inch diameter circular orbit. The seed flask is
then stored stationary at 4°C until it is ready to be used. The contents of this
flask are then used to inoculate (5% inoculum) 20 unbaffled 250 ml
Erlenmeyer flasks containing 40 ml of Medium No. 2. After 4 days incubation
at 28°C on a rotary shaking machine at 220 RPM with a 2 inch diameter
circular orbit, 19 of the flasks are harvested and pooled. The combined
fermentation broths
containing C-076 are filtered affording 500 ml of filtrate and 84 g of mycelia.
78 G of mycelia are extracted with 150 ml of acetone for ? hour with stirring
and the mixture filtered. The filter cake is washed with 50 ml of acetone and
the filtrate and washings are combined and concentrated to 46.5 ml 30 Ml of
the concentrate is adjusted to pH 4 with dilute hydrochloric acid and extracted
3 times with 30 ml portions of chloroform. The extracts are dried by filtering
through dry Infusorial Earth (Super-Cel) combined and concentrated to
dryness in vacuum. The oily residue of C-076 weighing 91.4 mg is dissolved in
chloroform sufficient to make 3 ml of solution which represents 1% of broth
volume. The C-076 (Abamectin) obtained in this recovery procedure is fully
active against N.dubius infections in mice. In addition, the chloroform
extraction achieved a 70 fold purification of C-076 from the whole broth.
brand name
(Merck)Avomec [Veterinary] (Merial); Bovitin [Veterinary] (Merial); Doratect [Veterinary] (Merial); Duomectin [Veterinary] (Merial); Duotin [Veterinary] (Merial); Endecto (Merck); Enzec (Merck); Enzek (Merck); Parafoil (Merck); Vertimil (Zectin (Merck).
Therapeutic Function
Antiparasitic
Hazard
A poison by ingestion. Moderately toxic by skin contact.
Safety Profile
A poison by ingestion.Moderately toxic by skin contact. When heated todecomposition it emits acrid smoke and irritating fumes.
Properties of Avermectin B1a
| Boiling point: | 940.9±65.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | ≥143.4 mg/mL in DMSO; ≥11.86 mg/mL in EtOH with ultrasonic; insoluble in H2O |
| form | Powder |
| pka | 12.42±0.70(Predicted) |
| color | White to light yellow |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Avermectin B1a (65195-55-3) |
Safety information for Avermectin B1a
Computed Descriptors for Avermectin B1a
Avermectin B1a manufacturer
Allas Chem Technologies Pvt Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 65195-55-3 Ivermectin EP Impurity A 98%View Details
65195-55-3 Ivermectin EP Impurity A 98%View Details
65195-55-3 -
 Ivermectin -5-methyl impurity 65195-55-3 98%View Details
Ivermectin -5-methyl impurity 65195-55-3 98%View Details
65195-55-3 -
 Ivermectin impurity A 97%View Details
Ivermectin impurity A 97%View Details
65195-55-3 -
 Avermectin 95% CAS 65195-55-3View Details
Avermectin 95% CAS 65195-55-3View Details
65195-55-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
