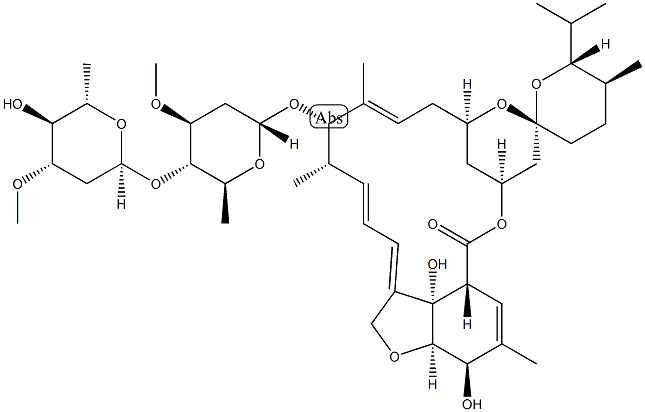
Avermectin A1a, 5-O-demethyl-22,23-dihydro-
- CAS NO.:71827-03-7
- Empirical Formula: C48H74O14
- Molecular Weight: 875.09
- EINECS: 276-046-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-19 16:27:22
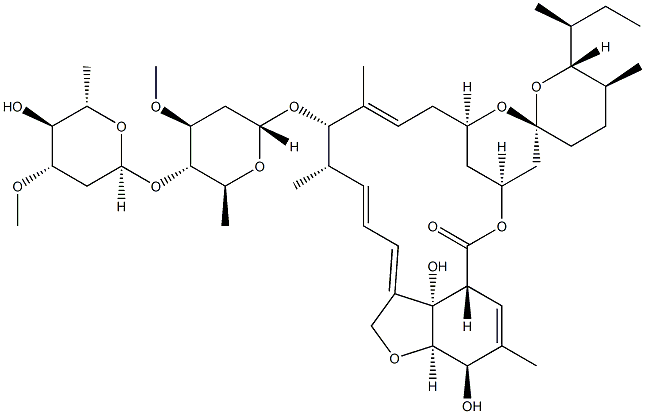
What is Avermectin A1a, 5-O-demethyl-22,23-dihydro-?
Description
Ivermectin B1a is the main component (>80%) of the anthelmintic ivermectin, which also contains ivermectin B1b (<20%; ). It produces antiparasitic activity by binding to glutamate-gated chloride channels expressed on nematode neurons and pharyngeal muscle cells, inducing irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function (EC50 = 104 nM). Formulations containing ivermectin inhibit replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Vero/hSLAM cells.
The Uses of Avermectin A1a, 5-O-demethyl-22,23-dihydro-
Ivermectin B1a-D2 is a deuterium labelled analogue of Ivermectin B1a (I940815), which is a major component of Ivermectin (I940800), a semi-synthetic derivative of Abamectin; consists of a mixture of not less than 80% component B1a and not more than 20% component B1b. Antihelmintic (Onchocerca). An invitro inhibitor of SARS-CoV-2/ Covid-19.
Definition
ChEBI: A macrocyclic lactone that is avermectin B1a in which the double bond present in the spirocyclic ring system has been reduced to a single bond. It is the major component of ivermectin.
References
[1] j. wolsetnholme and a. t. rogers. glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. parasitology131 suppl, s85-s95 (2005).
[2] s. gaisser, l. kellenberger, a. l. kaja, et al. direct production of ivermectin-like drugs after domain exchange in the avermectin polyketide synthase of streptomyces avermitilis atcc31272. organic & biomolecular chemistry 1(16), 2840-2847 (2003).
[3] j. p. arena, k. k. liu, p. s. paress, et al. the mechanism of action of avermectins in caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. journal of parasitology 81, 286-294 (1995).
Properties of Avermectin A1a, 5-O-demethyl-22,23-dihydro-
| Melting point: | 155-157° |
| Boiling point: | 717.97°C (rough estimate) |
| Density | 1.0683 (rough estimate) |
| refractive index | 1.6130 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 12.42±0.70(Predicted) |
| color | white |
Safety information for Avermectin A1a, 5-O-demethyl-22,23-dihydro-
Computed Descriptors for Avermectin A1a, 5-O-demethyl-22,23-dihydro-
New Products
Ethyl 3,3- diethoxypropionate 7-Bromo-2-chloroquinoxaline N-Benzyl-3-hydroxypiperidine 2-(4-bromophenyl)-2-methylpropanoic acid Benzylacetoacetate Radiator Flux Zinc Chloride Solution (All Grades) Phenylazomalononitrile 2-Fluoro-6-iodobenzoic acid 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 2,3-Difluoro-6-methoxybenzyl Chloride 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Acetyl-meldrum's acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II BromideRelated products of tetrahydrofuran

You may like
-
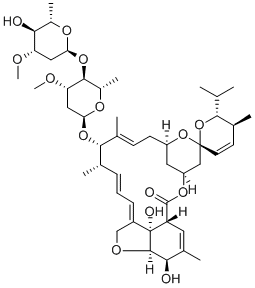
 71827-03-7 Ivermectin 2-epimer (Dihydro Avermectin B1a) 98%View Details
71827-03-7 Ivermectin 2-epimer (Dihydro Avermectin B1a) 98%View Details
71827-03-7 -
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 4'-Benzyloxy-2-bromopropiophenone 98%View Details
4'-Benzyloxy-2-bromopropiophenone 98%View Details
35081-45-9 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 29676-71-9 99%View Details
29676-71-9 99%View Details
29676-71-9 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1
