atpenin A5
- CAS NO.:119509-24-9
- Empirical Formula: C15H21Cl2NO5
- Molecular Weight: 366.24
- MDL number: MFCD01664873
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
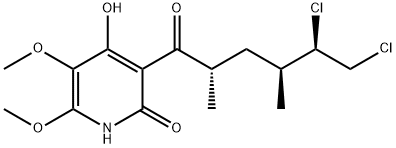
What is atpenin A5?
Description
Enantioselective total synthesis of atpenin A5, a potent mitochondrial complex II (succinate-ubiquinone oxidoreductase) inhibitor, has been achieved by a convergent approach through a coupling reaction between 5-iodo-2,3,4,6-tetraalkoxypyridine and a side-chain aldehyde. The two key segments were synthesized through ortho-metalation/boronation with (MeO)3B/oxidation with mCPBA, ortho-iodination, halogen dance reaction, Sharpless epoxidation and regioselective epoxide-opening reaction.
The Uses of atpenin A5
Atpenin A5 is a mitochondrial complex II inhibitor able to protect against cardiac-reperfusion.
What are the applications of Application
Atpenin A5 is a mitochondrial complex II inhibitor able to protect against cardiac-reperfusion
Definition
Atpenin A5 belongs to a class of antifungal antibiotics isolated from Penicillium sp. The atpenins are effective against filamentous fungi, particularly Trichophyton sp., and are specific and potent inhibitors of the succinate-ubiquinone reducatase activity of the mitochondrial complex II. Atpenin A5 is the most effective inhibitor against complex II from all the atpenins and has been shown to protect against cardiac-reperfusion injury in rat studies through the stimulation of mitochondrial KATP channels. Atpenin A5 is an inhibitor of SDHB, SDHC and SDHD.
Biological Activity
Mitochondrial complex II (succinate dehydrogenase or succinate:ubiquinone oxidoreductase) is a functional member of the Krebs cycle and the aerobic respiratory chain that couples the oxidation of succinate to fumarate with the reduction of quinone to quinol. Atpenin A5, an antifungal antibiotic isolated from Penicillium sp. found in soil, is a highly specific ubiquinone-binding site inhibitor of succinate dehydrogenase (IC50s = 12 and 3.7 nM in nematode and mammalian mitochondria, respectively, versus IC50s > 100 μM for inhibition of complex I and complex III enzymes). Atpenin A5 has cardioprotective effects against simulated ischemia-reperfusion injury in cardiomyocytes. Several mechanisms through which this occurs, including activation of mitochondrial ATP-sensitive potassium channels or modulation of mitochondrial reactive oxygen species generation, have been proposed.
in vitro
Highly selective, potent ubiquinone-binding site mitochondrial complex II inhibitor (IC50 values are 12 and 3.7 nM for nematode and mammalian mitochondria respectively). Stimulates mitochondrial KATP channels. Shows anti-ischemic and cardioprotective effects in vivo.
storage
+4°C
Properties of atpenin A5
| Melting point: | 83-85℃ |
| Boiling point: | 538.3±50.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chlroform (Slightly), Ethanol (Slightly), Methanol (Slightly, Sonicated) |
| form | White to off-white powder. |
| pka | 4.50±1.00(Predicted) |
| color | White to Off-White |
Safety information for atpenin A5
Computed Descriptors for atpenin A5
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(-)-4-Hydroxy-5,6-dimethoxy-3-[(E)-2-methyl-1-oxo-4-hexenyl]pyridine-2(1H)-one](https://img.chemicalbook.in/CAS/GIF/137813-88-8.gif)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1