Asunaprevir
- CAS NO.:630420-16-5
- Empirical Formula: C35H46ClN5O9S
- Molecular Weight: 748.29
- MDL number: MFCD27987900
- EINECS: 681-328-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
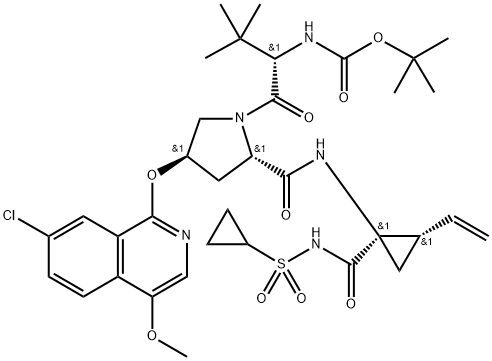
What is Asunaprevir?
Absorption
In preclinical studies, asunaprevir showed a high liver-to-plasma AUC ratio. It is rapidly absorbed within 30 minutes of administration. Clinical pharmacokinetic studies showed a Tmax of 2-4 hours. The pharmacokinetic profile act in a dose-proportional manner and in a dose of 100 mg the steady-state Cmax and AUC was 572 ng/ml and 1887 ng x h/mL. The absolute bioavailability is reported to be 9.3%. The absorption of asunaprevir is increased with food.
Toxicity
Toxicity studies showed no carcinogenic nor genotoxic potential related to asunaprevir. In the case of overdose, clinical studies reported no unexpected adverse events. Asunaprevir had no effects on fertility in preclinical studies. It has been shown that asunaprevir gets localized in the GI tract and liver and thus, increased hepatic transaminases were observed as well as changes in iron metabolism and decreased serum proteins. These effects are not progressive and asunaprevir was generally well tolerated.
Description
Sold under the trade name Sunvepra®, asunaprevir received approval in Japan as part of a combination treatment for the hepatitis C virus (HCV). Working in concert with daclatasvir (IX) (vide infra), asunaprevir is a unique treatment for HCV, as it is free from both interferon and ribavirin and is administered orally. This direct-acting anti-viral, which was developed by Bristol–Myers Squibb (BMS), works as an NS3/4A protease inhibitor, representing a valuable treatment option for patient populations who are unable to receive, or do not respond to, the standard course of treatment—peginterferon/ribavirin.
Description
Asunaprevir is an inhibitor of the hepatitis C non-structural 3/4A serine protease, which is required for viral replication. It is a direct-acting inhibitor of hepatitis C virus (HCV). It is commonly used in combination with daclatasvir and beclabuvir, which are also direct-acting HCV inhibitors.
The Uses of Asunaprevir
Asunaprevir is an inhibitor of the viral enzyme serine protease HCV NS3. Also functions as a second generation NS3/4A protease inhibitor used in the treatment of hepatitis C.
Background
Asunaprevir, also named BMS-650032, is a potent hepatitis C virus (HCV) NS3 protease inhibitor. It has been shown to have a very high efficacy in dual-combination regimens with daclatasvir in patients chronically infected with HCV genotype 1b. It was developed by Bristol-Myers Squibb Canada and approved by Health Canada on April 22, 2016. The commercialization of asunaprevir was cancelled one year later on October 16, 2017.
Indications
Asunaprevir is indicated in combination with other agents for the treatment of chronic hepatitis C in adult patients with hepatitis C virus genotypes 1 or 4 and compensated liver cirrhosis.
Hepatitis C is a liver disease caused by the hepatitis C virus. The chronic state of this condition accounts for 60-80% of the cases from which the risk of cirrhosis of the liver within 20 years is of around 15-30%. The genotype 1 is the most common type of hepatitis C in the United States and the most difficult to treat.
What are the applications of Application
Asunaprevir is an inhibitor of the viral enzyme serine protease HCV NS3
Definition
ChEBI: Asunaprevir is an oligopeptide.
Pharmacokinetics
Studies in vitro demonstrated a significant antiviral activity in HCV replicon cell systems with an EC50 of 4nm and 1nm against the HCV genotype 1a and 1b respectively. These studies showed a limited activity against the genotypes 2 and 3. This property makes asunaprevir a highly selective anti-HCV agent that is not effective against HCV closely related virus. Asunaprevir produce robust declines in HCV RNA levels in patients with HCV genotype 1 infection.In clinical studies, it has been shown that asunaprevir is well-tolerated and the mean maximum HCV RNA level reduction from baseline was of approximately 2.87 log10 IU/ml.
Monotherapy clinical studies with asunaprevir showed a mean maximum decline of HCV RNA in the range of 0.28-2.87 log10 IU/ml when administered in increasing doses from 10-600 mg. When asunaprevir was used as a combination product, it was possible to obtain a sustained virological response (aviremia 24 weeks after completion of therapy) in 83-92% of the patients.
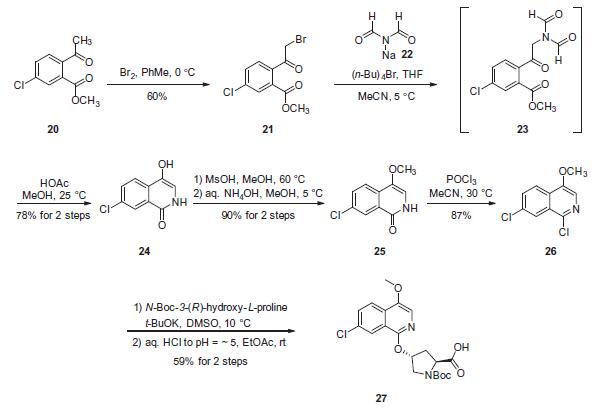
Synthesis
The preparation of chloroquinoline 27 began with bromination
of commercially available acetophenone 20, which was carefully
carried out on over 1 kg scale in a reactor equipped with an HBr
scrubbing mechanism (bubbler outfitted) to furnish a-bromoketone
21. Next, nucleophilic displacement of the bromide
using sodium diformamide (22) under phase transfer
conditions led to the putative intermediate 23. This system underwent
in situ deprotection of the diformamide functionality followed
by cyclization under mildly acidic conditions to arrive at
isoquinolone 24 in 78% yield across the two-step, one-pot
sequence. Next, methylation of the hydroxyl group at C-4 was carried
out using methanolic methanesulfonic acid at elevated temperatures
followed by treatment with aqueous ammonium
hydroxide to quench any excess acid. Subsequent treatment with
phosphorous oxychloride furnished dichloroisoquinoline 26 in
good yield. A nucleophilic aromatic substitution reaction employing potassium t-butoxide was used to establish an arylpyrrolidino
ether linkage and, upon workup with mildly acidic conditions,
the proline derivative 27 emerged in 59% yield.
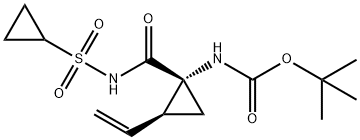
Although the synthesis of asunaprevir vinyl cyclopropane subunit
35 has been described by researchers at BMS, it is interesting
to note that this subunit, or structural derivatives of 35, have been found within a number of other antiviral drugs, particularly those
which target inhibition of NS3 protease as its mechanism of action.
For example, researchers at Boehringer-Ingelheim have described a
scale synthesis of (1R,2S)-1-amino-2-vinylcyclopropane carboxylic
acid (des-N-tert-butoxycarbonyl (Boc) 32; also referred
to as ??vinyl-ACCA?ˉ) as a means to incorporate this subunit into that
company?ˉs antiviral agent BILN 2061. A macrocyclic version of
this same cyclopropane-containing system is found in the antiviral
agents paritaprevir hydrate (XXVII) developed by Enanta and Abbvie,
and a saturated version in vaniprevir (XXXVII) developed by
Merck and described later in this review (vide infra). The preparation
of 32 described by Beaulieu and co-workers was performed on
pilot-plant scale and the details surrounding the conversion of 32
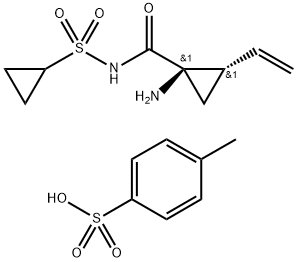
to 35 are described by the BMS patent. Beginning with commercially
available methyl glycine HCl (28), condensation with benzaldehyde
in the presence of a dehydrating reagent (trimethyl
orthoformate) and base led to the transient imine 29. This
underwent a highly diastereoselective alkylation reaction whereby
treatment with lithium t-butoxide followed by subjection to 1,4-
dibromo-2-butene facilitated an SN2¨CSN20 reaction. Subsequent
acidification and Boc protection resulted in 30-rac as a racemate
that existed as a single diastereomer having the vinyl and the ester
groups in a cis configuration. The authors postulate that the lithium
enolate intermediate which forms upon the initial alkylation binds the proximal bromine atom which accounts for the stereoselectivity
of the reaction. Next, selective saponification of the undesired
enantiomer 31b took place via treatment with acalase 2.4 L, which
is a remarkably versatile and relatively inexpensive enzyme capable
of resolving a wide variety of racemic esters,49 under basic
conditions. This enzymatic resolution returned a 49% yield of the
desired ester 31a (theoretical 50%), and a simple aqueous workup
removed the undesired acid 31b. Methyl ester 31a was then
saponified using methanolic lithium hydroxide to give 32, which
was subsequently coupled with cyclopropanesulfonamide 33 to
deliver 34 in excellent yield. Next, removal of the Boc group using
TFA followed by salt formation with ethereal HCl delivered the key
cyclopropane subunit 35.

Cyclopropyl amine 35 was subjected to proline
derivative 27 under conventional amide bond-forming conditions.
This was followed by removal of the pyrrolidine nitrogen Boc
group with acid and subsequent coupling with N-Boc-3-methyl
valine (37) to deliver asunaprevir (IV) in good yield for each of
these steps.

Enzyme inhibitor
This highly selective HCV antiviral (FW = 748.29 g/mol; CAS 630420-16- 5; Symbol: ASV), also named BMS-650032, targets NS3 protease, a serine proteinase required for Hepatitis C Virus (HCV) polyprotein processing, showing good antiviral activity against replicons based on HCV Genotype 1a (EC50 = 4 nM), Genotype 1b (EC50 = 1.2 nM), Genotype 4 (EC50 = 1.8 nM), Genotype 5 (EC50 = 1.7 nM), and Genotype 6 (EC50 = 0.9 nM). It is far less effective against Genotype 2 (EC50 = 67 nM) and Genotype 3 (EC50 = >1100 nM). Asunaprevir is a peptidomimetic that occupies the active site, inhibiting the HCV NS3/4A serine protease, with little or no action against related viruses. The average Ki for ASV is approximately 0.4 nM and 0.2 nM for Genotype 1a and Genotype 1b, respectively. Its acylsulfonamide moeity interacts noncovalently with the NS3 protease – unlike telaprevir’s α-ketoamide moiety, which is linked covalently to NS3/4A’s catalytic serine and reverses slowly over time. ASV exhibits an excellent selectivity index (>40,000x) against all serine/cysteine proteases evaluated, including human leukocyte elastase, porcine pancreatic elastase, and three members of the chymotrypsin family. Although ASV shows a low barrier to resistance, it can be combined with daclatasvir to achieve a very high rate of viral eradication in both na?ve and treatment-experienced patients, showing a sustained virological response rate of 80%-90%.
Metabolism
Asunaprevir is metabolized by the liver. The metabolism is mainly marked by oxidative reactions mediated by the activity of CYP3A. Asunaprevir seems to weakly induce its own metabolism and from the circulating dose, just about 5% of the administered dose is formed by metabolites. The metabolites of asunaprevir are formed after mono- and bis-oxidation, N-dealkylation, loss of isoquinoline ring and O-demethylation. All the metabolic reactions form about 15 metabolites and studies have reported that the main metabolic activity is performed by CYP3A4 and CYP3A5 with some minor activity from CYP2A6, CYP2B6, CYP2C9, CYP2C19 and CYP2D6.
storage
Store at -20°C
References
[1] scola pm, sun lq, wang ax, chen j, sin n, venables bl, sit sy, chen y, cocuzza a, bilder dm, d'andrea sv, zheng b, hewawasam p, tu y, friborg j, falk p, hernandez d, levine s, chen c, yu f, sheaffer ak, zhai g, barry d, knipe jo, han yh, schartman r, donoso m, mosure k, sinz mw, zvyaga t, good ac, rajamani r, kish k, tredup j, klei he, gao q, mueller l, colonno rj, grasela dm, adams sp, loy j, levesque pc, sun h, shi h, sun l, warner w, li d, zhu j, meanwell na, mcphee f. the discovery of asunaprevir (bms-650032), an orally efficacious ns3 protease inhibitor for the treatment of hepatitis c virus infection. j med chem. 2014 mar 13;57(5):1730-52.
[2] mcphee f, sheaffer ak, friborg j, hernandez d, falk p, zhai g, levine s, chaniewski s, yu f, barry d, chen c, lee ms, mosure k, sun lq, sinz m, meanwell na, colonno rj, knipe j, scola p. preclinical profile and characterization of the hepatitis c virus ns3 protease inhibitor asunaprevir (bms-650032). antimicrob agents chemother. 2012 oct;56(10):5387-96.
Properties of Asunaprevir
| Melting point: | 149-150oC |
| Density | 1.37 |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 4.49±0.40(Predicted) |
| color | White to Off-White |
Safety information for Asunaprevir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Asunaprevir
Asunaprevir manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Asunaprevir 98% (HPLC) CAS 630420-16-5View Details
Asunaprevir 98% (HPLC) CAS 630420-16-5View Details
630420-16-5 -
 Asunaprevir CAS 630420-16-5View Details
Asunaprevir CAS 630420-16-5View Details
630420-16-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1