Astemizole
Synonym(s):1-(4-Fluorobenzyl)-2-(1-[4-methoxyphenethyl]piperidin-4-yl)aminobenzimidazole
- CAS NO.:68844-77-9
- Empirical Formula: C28H31FN4O
- Molecular Weight: 458.57
- MDL number: MFCD00153919
- EINECS: 272-441-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
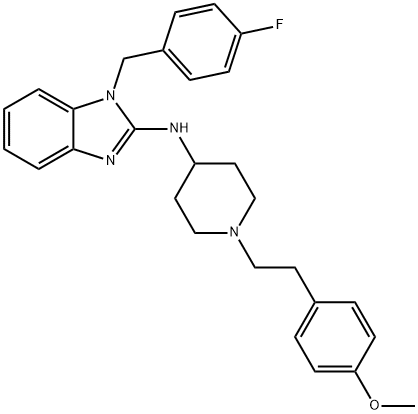
What is Astemizole?
Absorption
Rapidly absorbed from the gastrointestinal tract.
Toxicity
LD50=2052mg/kg in mice
Description
Astemizole belongs to the second-generation class of non-sedating, non-anticholinergic antihistamines. Its non-sedating properties appear to result from its poor penetration of the blood brain barrier. As a result it shows no potentiation of CNS depressants, including alcohol. Its long half-life allows once-daily dosing.
Chemical properties
Crystalline Solid
Originator
Janssen (Belgium)
The Uses of Astemizole
Astemizole is used for preventing and treating severe seasonal and chronic allergic rhinitis, allergic conjunctivitis, hives, Quinke’s edema, other allergic conditions and dermatitis. Synonyms of this drug are hismanal, histazol, and others.
The Uses of Astemizole
Nonsedating-type histamine H1-receptor antagonist. Potential for combination therapy with antivancer drugs such as doxorubicin in resistant leukemia. Antihistaminic
The Uses of Astemizole
scabicide
Background
Astemizole is a long-acting, non-sedating second generation antihistamine used in the treatment of allergy symptoms. It was withdrawn from market by the manufacturer in 1999 due to the potential to cause arrhythmias at high doses, especially when when taken with CYP inhibitors or grapefruit juice.
Indications
Astemizole was indicated for use in the relieving allergy symptoms, particularly rhinitis and conjunctivitis. It has been withdrawn from the market however due to concerns of arrhythmias.
What are the applications of Application
Astemizole is a potent and selective H1-receptor antagonist that displays antihistamine and antimalarial activity
Definition
ChEBI: A piperidine compound having a 2-(4-methoxyphenyl)ethyl group at the 1-position and an N-[(4-fluorobenzyl)benzimidazol-2-yl]amino group at the 4-position.
Manufacturing Process
A mixture of 2.3 parts of 2-(4-methoxyphenyl)ethyl methanesulfonate, 4.9 parts of 1-[(4-fluorophenyl)methyl]-N-(4-piperidinyl)-1H-benzimidazol-2- amine dihydrobromide, 3.2 parts of sodium carbonate, 0.1 part of potassium iodide and 90 parts of N,N-dimethylformamide is stirred overnight at 70°C. The reaction mixture is poured onto water. The product is extracted with methylbenzene. The extract is washed with water, dried, filtered and evaporated. The residue is purified by column-chromatography over silica gel using a mixture of trichloromethane and methanol (98:2 by volume) as eluent. The pure fractions are collected and the eluent is evaporated. The residue is crystallized from 2,2'-oxybispropane, yielding 2.2 parts (48%) of 1- (4-fluorophenylmethyl)-N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1Hbenzimidazol- 2-amine, MP 149.1°C.
brand name
Hismanal (Janssen);Alermizol;Astezol;Astol;Histamanal;Novo-nastizol;EISMANAL.
Therapeutic Function
Antiallergic, Antihistaminic
World Health Organization (WHO)
The first clinically interesting histamine ti-antagonists were introduced in the late forties and early fifties. Several histamine ti-antagonists have a similar cardiac effect to that seen with astemizole and terfenadine. Serious cardiovascular adverse reactions have been reported when used concomitantly with imidazole antifungals and macrolide antibiotics.
Biological Activity
Orally active, potent histamine H 1 antagonist (IC 50 = 4 nM) that displays 20-fold, > 250-fold and > 250-fold selectivity over 5-HT, dopamine and muscarinic acetylcholine receptors respectively. Exhibits antimalarial activity in multidrug resistant strains in vitro (IC 50 = 227 - 734 nM). Also potent hERG K + channel blocker (IC 50 = 0.9 nM) that displays cardiotoxicity in vivo .
Biochem/physiol Actions
Astermizole is a potent hERG potassium channel blocker (IC50 of 0.9 nM) and may used as a pharmacological chaperone to correct folding defects and restore protein function for some mutated forms of hERG channels. It has also been studied for treatment of malaria, hERG and hEAG channel function in cancer and as a second generation antihistamine H-1 antagonist.
Pharmacokinetics
Astemizole is a second generation H1-receptor antagonist. It does not significantly cross the blood brain barrier and therefore does not cause drowsiness or CNS depression at normal doses.
Safety Profile
Poison by subcutaneous andintravenous routes. Moderately toxic by ingestion. Humansystemic effects by ingestion: arrhythmias, coma, nauseaor vomiting, somnolence. When heated to decompositionit emits toxic fumes of F?? and NOx.
Synthesis
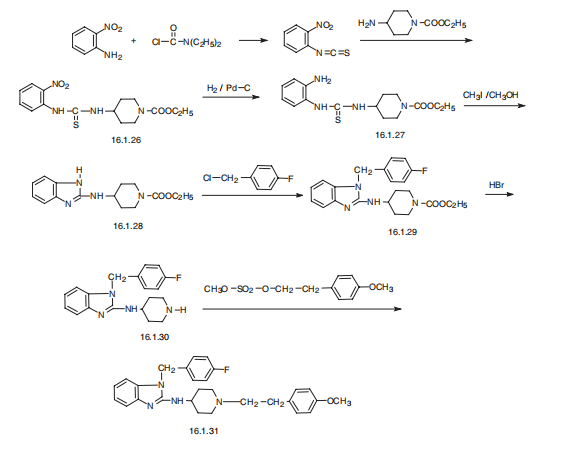
Astemizole, 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl) ethyl]-4- piperidinyl]-benzimidazol-2-amine (16.1.31), is synthesized in a multi-stage synthesis from 1-carbethoxy-4-aminopiperidine and 2-nitroisothiocyanobenzol, from which a derivative of thiourea (16.1.26) is synthesized upon their reaction. The nitro group of the product is reduced and the further S-methoxided. In reaction conditions intermolecular cyclization into a derivative of benimidazol, N-[1-[2-(4-carethoxy)]-4-piperidinyl]benzimidazol-2-amine (16.1.28) occurs. The obtained aminobenzimidazole derivative is alkylated with 4-fluorobenzylchoride into 1-[(flurophenyl)methyl]-N-[1-[2-(4-carethoxy)]-4-piperidinyl] benzimidazol- 2-amine (16.1.29). The carbethoxyl group of the resulting compound (16.1.29) is hydrolyzed by hydrobromic acid, forming a non-substituted on the nitrogen atom derivative of piperidine (16.1.30), the alkylation of which with 2-(4-methoxyphenyl)ethylmetanesulfonate leads to the formation of astemizole (16.1.31).
Metabolism
Almost completely metabolized in the liver and primarily excreted in the feces.
storage
Store at +4°C
References
1) Richards et al. (1984), Astemizole. A review of its pharmacodynamic properties and therapeutic efficacy; Drugs, 28 38 2) Laduron et al. (1982), In vitro and in vivo binding characteristics of a new long-acting histamine H1 antagonist, astemizole; Mol. Pharmacol., 21 294
Properties of Astemizole
| Melting point: | 172.9°C |
| Boiling point: | 627.3±65.0 °C(Predicted) |
| Density | 1.1587 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: >20mg/mL |
| form | powder |
| pka | pKa 4.85(H2O t=25.0 I=0.025) (Uncertain);8.69(H2O t=25.0 I=0.025) (Uncertain) |
| color | Crystals |
| Water Solubility | It is soluble in DMSO (25 mg/ml ), ethanol (25 mg/ml), chloroform, methanol, and water (partly miscible). |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for Astemizole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Astemizole
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Astemizole 99.00% CAS 68844-77-9View Details
Astemizole 99.00% CAS 68844-77-9View Details
68844-77-9 -
 Astemizole CAS 68844-77-9View Details
Astemizole CAS 68844-77-9View Details
68844-77-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8