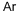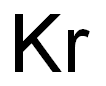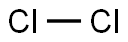astatine
- Empirical Formula: At2
- Molecular Weight: 420

What is astatine?
Physical properties
Astatine is located just below iodine, which suggests that it should have some of the samechemical properties as iodine, even though it also acts more like a metal or semimetal thandoes iodine. It is a fairly heavy element with an odd atomic number, which assisted chemistsin learning more about this extremely rare element. The 41 isotopes are man-made in atomicreactors, and most exist for fractions of a second. The element’s melting point is about 302°C,its boiling point is approximately 337°C, and its density is about 7g/cm3.
Isotopes
All 41 isotopes of astatine are radioactive, with half-lives ranging from 125nanoseconds to 8.1 hours. The isotope As-210, the most stable isotope with an 8.1-hour half-life, is used to determine the atomic weight of astatine. As-210 decays byalpha decay into bismuth-206 or by electron capture into polonium-210.
Origin of Name
From the Greek word astatos, which means “unstable.” All of its isotopes are unstable.
Occurrence
Chemists of the early twentieth century tried to find the existence of element 85, whichwas given the name “eka-iodine” by Mendeleev in order to fill the space for the missing elementin the periodic table. Astatine is the rarest of all elements on Earth and is found in onlytrace amounts. Less than one ounce of natural astatine exists on the Earth at any one time.There would be no astatine on Earth if it were not for the small amounts that are replenishedby the radioactive decay process of uranium ore. Astatine produced by this uranium radioactivedecay process soon decays, so there is no long-term build up of astatine on Earth. Theisotopes of astatine have very short half-lives, and less than a gram has ever been produced forlaboratory study.
History
Synthesized in 1940 by D. R. Corson, K. R. MacKenzie, and E. Segre at the University of California by bombarding bismuth with alpha particles. The longest-lived isotope, 210At, has a half-life of only 8.1 hours. Thirty-six other isotopes and isomers are now known. Minute quantities of 215At, 218At, and 219At exist in equilibrium in nature with naturally occurring uranium and thorium isotopes, and traces of 217At are in equilibrium with 233U and 239Np resulting from interaction of thorium and uranium with naturally produced neutrons. The total amount of astatine present in the Earth’s crust, however, is probably less than 1 oz. Astatine can be produced by bombarding bismuth with energetic alpha particles to obtain the relatively long-lived 209–211At, which can be distilled from the target by heating it in air. Only about 0.05 μg of astatine has been prepared to date. The “time of flight” mass spectrometer has been used to confirm that this highly radioactive halogen behaves chemically very much like other halogens, particularly iodine. The interhalogen compounds AtI, AtBr, and AtCl are known to form, but it is not yet known if astatine forms diatomic astatine molecules. HAt and CH3At (methyl astatide) have been detected. Astatine is said to be more metallic that iodine, and, like iodine, it probably accumulates in the thyroid gland.
Characteristics
Astatine is the heaviest and densest of the elements in group 17 (VIIA). It is difficult todetermine the chemical and physical properties and characteristics of astatine because it ispresent in such small quantities that exist for extremely short periods of time. Many of itscharacteristics are inferred through experiments rather than by direct observations.
The Uses of astatine
In water solution astatine resembles iodine in some of its chemical and physical properties.Both are powerful oxidizing agents.
It has limited use in medicine as a radioactive source. It concentrates in the thyroid glandjust like iodine, which makes it a useful radioisotope tracer.
Because of its isotopes’ short half-lives and its scarcity, astatine has few practical uses outsideof the laboratory for research, where less than a gram has ever been produced.
Definition
Nonmetallic element of atomic number 85. Group VIIA of periodic table, aw 211. Heaviest member of the halogen family, has 20 isotopes, all radioactive; derived by α-bombardment of bismuth. The two most stable isotopes have halflives of approximately 8 hours. Astatine occurs in nature to the extent of approximately one ounce in the entire earth’s crust. Like iodine, it concentrates in the thyroid gland. Its use in medicine is still experimental.
Definition
A radioactive
element belonging to the halogen group. It
occurs in minute quantities in uranium
ores. Many short-lived radioisotopes are
known, all alpha-particle emitters.
Symbol: At; m.p. 302°C (est.); b.p.
337°C (est.); p.n. 85; most stable isotope
210At (half-life 8.1 hours).
Definition
astatine: Symbol At. A radioactivehalogen element; a.n. 85; r.a.m. 211;m.p. 302°C; b.p. 337°C. It occurs naturallyby radioactive decay from uraniumand thorium isotopes. Astatineforms at least 20 isotopes, the moststable astatine–210 has a half-life of8.3 hours. It can also be produced byalpha bombardment of bismuth–200.Astatine is stated to be more metallicthan iodine; at least 5 oxidationstates are known in aqueous solutions.It will form interhalogen compounds,such as AtI and AtCl. Theexistence of At2 has not yet been established.The element was synthesizedby nuclear bombardment in1940 by D. R. Corson, K. R. MacKenzie,and E. Segré at the University ofCalifornia.
Hazard
The major hazard is from the radiation of astatine’s isotopes. However, given that theseisotopes have very short half-lives, they do not pose a great long-term danger. Even so, astatineis considered a dangerous element that is a radioactive poison and carcinogen. It has beendemonstrated that astatine causes cancer in laboratory animals.
Safety information for astatine
New Products
p-Toluenesulfonyl hydrazide N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one ELECTROLYTIC IRON POWDER 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 1-(2,4-DICHLOROPHENYL) ETHANAMINE 4-Bromopyrazole 1-Boc-4-cyanopiperidine 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone Cycloleucine 1-Aminocyclobutanecarboxylic acid 1-Amino-1-cyclohexanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride TETRABUTYLAMMONIUM CYANIDE Ethyl 2-cyano-2-(hydroxyimino)acetate (S)-1-Benzyl 2-methyl pyrrolidine-1,2-dicarboxylate 2-Amino-3-nitrobenzoic acid methyl ester N1,N2-Dibenzylethane-1,2-diamine 3-(4-Morpholinyl)aniline 4-methoxy-3,5-dinitropyridineRelated products of tetrahydrofuran
You may like
-
![Methyl (E) -2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylate](https://img.chemicalbook.in//Content/image/CP5.jpg) Methyl (E) -2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylateView Details
Methyl (E) -2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxyacrylateView Details
131860-97-4 -
 4-N Butyl Resorcinol 18979-61-8 NLT 98%View Details
4-N Butyl Resorcinol 18979-61-8 NLT 98%View Details
18979-61-8 -
 719-22-2 2,6- Di-Tert-Butyl-p-Benzoquinone NLT 98%View Details
719-22-2 2,6- Di-Tert-Butyl-p-Benzoquinone NLT 98%View Details
719-22-2 -
 1,3 Cyclohexane dione NLT 98%View Details
1,3 Cyclohexane dione NLT 98%View Details
504-02-9 -
 Ethylhexyl Triazone 88122-99-0 NLT 98%View Details
Ethylhexyl Triazone 88122-99-0 NLT 98%View Details
88122-99-0 -
 52-52-8 Cycloleucine 98+View Details
52-52-8 Cycloleucine 98+View Details
52-52-8 -
 2756-85-6 98+View Details
2756-85-6 98+View Details
2756-85-6 -
 1-Aminocyclobutanecarboxylic acid 98+View Details
1-Aminocyclobutanecarboxylic acid 98+View Details
22264-50-2