Arbidol
- CAS NO.:131707-25-0
- Empirical Formula: C22H25BrN2O3S
- Molecular Weight: 477.41
- MDL number: MFCD01326495
- EINECS: 814-571-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
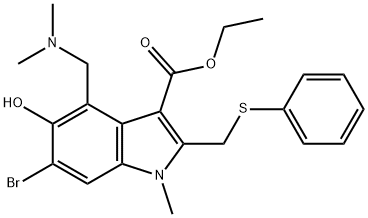
What is Arbidol?
Description
Arbidol, or umifenovir, an indole-derivative with broad-spectrum activity against both enveloped and non-enveloped viruses,was initially approved in China and Russia for the treatment of influenza A and B.Arbidol is believed to block the entry of influenza virus (A and B) into the host cells by increasing the stability of the hemagglutinin (HA) and hampering low pH reorganizations necessary for fusion machinery of hemagglutinin with the membrane.Arbidol could interfere with advanced stages of the viral life cycle, in that it is capable of interacting with both viral proteins and lipids.Regarding its structure, the presence of amine in position 4 and the hydroxyl moiety in position 5 is crucial for its antiviral activity. It is reported that 40% of the drug could be excreted unchanged after the administration while its half-life is between 17 and 21h.
The Uses of Arbidol
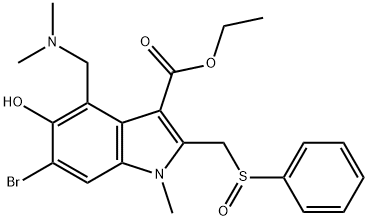
Umifenovir is an intermediate in synthesizing Arbidol Sulfoxide (A766005), a metabolite of Arbidol (A766000) which is a medicinal agent for treating viral infections.
Definition
ChEBI: Umifenovir is an indolyl carboxylic acid.
Biological Activity
Arbidol Arbidol is a broad spectrum antiviral. Displays activity against a range of respiratory and hepatitis viruses including influenza A, B and C, adenovirus, and hepatitis B and C. Increases stability of influenza viral hemagglutinins (HA) and prevents low-pH HA transition so inhibiting viral fusion. Also inhibits SARS-CoV-2 infection in vitro (IC50 = 4.11 μM).
Mechanism of action
Probable Mechanism of Action
lt is hypothesized that arbidol inhibits the contact between the virus and host cell that further prevents the entry of the virus into host cells. It is also being predicted that arbidol also interferes with the late stages of virus cycle by interacting with lipids and proteins of the virus which are essential for the viral replication;probably arbidol binds with these essential components that further inhibit viral replication (Boriskin et al., 2008).
Umifenovir inhibits membrane fusion.Umifenovir prevents contact between the virus and target host cells. Fusion between the viral envelope (surrounding the viral capsid) and the cell membrane of the target cell is inhibited. This prevents viral entry to the target cell, and therefore protects it from infection.
Some evidence suggests that the drug’s actions are more effective at preventing infections from RNA viruses than infections from DNA viruses.
As well as specific antiviral action against both influenza A and influenza B viruses, umifenovir exhibits modulatory effects on the immune system. The drug stimulates a humoral immune response, induces interferon-production, and stimulates the phagocytic function of macrophages.
https://newdrugapprovals.org
Clinical Use
Arbidol in Treatment of covID-19
Although,arbidol is not approved by US-FDA for the treatment of cOVID-19,some pre-clinical and clinical studies have reported that it may be a very efficacious therapeutic molecule for COVID-19 (Deng et al.,2020; Wang et al.,2020).Arbidol has been included in the latest version of the guidelines issued by the National Health Commission (NHC) of the people's republic of China for the prevention, diagnosis, and treatment of novel coronavirus induced pneumonia at the recommended dose of 200 mg, 3 times/day for 10 days for adults (National Health Commission,China).
An in-vitro study claimed that arbidol is effective against COVID-19 at the concentration range of 10-30 uM (News: Abidol and darunavir can effectively inhibit corona virus (2020). A retrospective study conducted by Deng and colleagues reported that patients who received oral arbidol plus lopinavir/ritonavir showed improvement in clinical symptoms and reduced viral load as a compared to the patients who received lopinavir/ritonavir alone (Deng et al.,2020).A randomized multi-centre controlled clinical trial of arbidol in the patients with cOVID-19 is currently running in China (ChiCTR2000029573).
Besides this, one more study was conducted on 69 patients of COVID-19.In this study, it was reported that the administration of arbidol at the dose of 0.4 g,TID for 9 days improves the discharging rate from the hospital and also decreases the mortality rate of patients (Wang et al.,2020). In addition,another study also reported that arbidol is more superior to the combination therapy of lopinavir/ritonavir (Zhu et al.,2020).
Arbidol: a broad-spectrum antiviral compound that blocks viral fusion
Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein
Properties of Arbidol
| Boiling point: | 591.8±50.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 6.02±0.50(Predicted) |
| color | Pale Brown to Light Brown |
Safety information for Arbidol
Computed Descriptors for Arbidol
Arbidol manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Umifenovir 99%View Details
Umifenovir 99%View Details -
 131707-25-0 98%View Details
131707-25-0 98%View Details
131707-25-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8